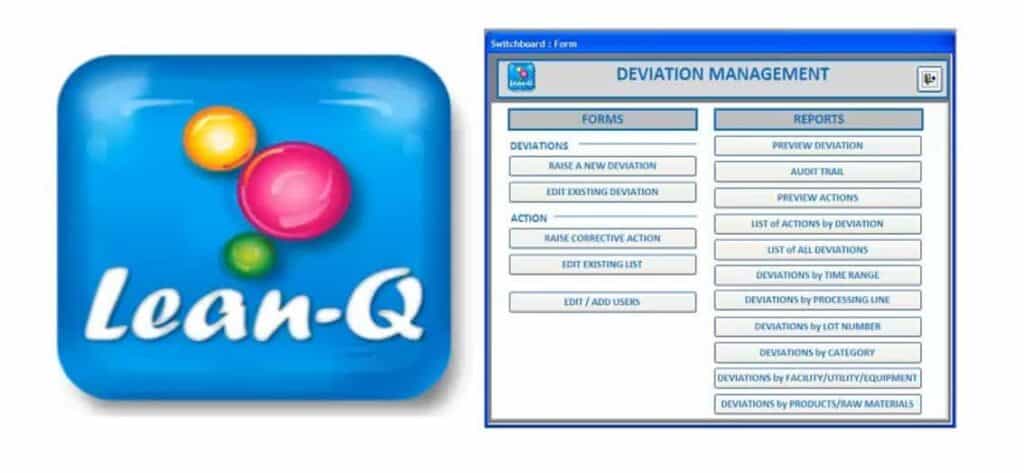
Deviation Management Module
The deviation management module is designed to initiate, investigate, review and approve deviation records by completing tasks sequentially tasks such as raising deviations with deviation details, deviation assessment, investigation information, root cause analysis, risk assessment, raising CAPA, final review & approval of the record. Deviation module has built in report queries for generating useful reports which can be saved in pdf format.
The operation of the module is GMP compliant since the designated initiator, investigator, reviewer and approver can complete their tasks and sign off electronically in real time. All saved information needs to be changed under a strict record change management system. The record change activities can be audited through an Audit Trail reporting.
Main Features :
- Unrestricted database. Easy to change and modify to reflect your own work flow and terminology
- Low cost and efficient Access based software for small and medium size GMP manufacturing enterprise
- Effortless installation for Windows Office Operating systems. Just Download and use in the next minute
- Designed to comply with management of GMP records
- Save time – save money – Less work. No more paper based quality investigations needed
- Electronic tracking, trending and reporting of quality investigations
- Step-by-step logical investigation workflow.
- Record must be raised, investigated, risk assessed, reviewed, approved and closed by the designated responsible users
- No escape from accountability. Lean-Q has a built in Audit Trail and permanent activity history checks
- Increased visibility with realtime electronic signatures on the records and reports
- Capable of selecting data from every area of a GMP manufacturing site
- User authentication controlled by the system Administrator
- Lean-Q facilitates enhanced flexibility and communication between functional teams
- Module specific GMP reporting system. Can be used for reporting, analysis and trending of investigations
