What is analytical laboratory management in pharmaceutical?
- Kazi
- Last modified: March 13, 2025
Analytical laboratories in the pharmaceutical industry are highly regulated work environments. Effective laboratory management is key to compliance with regulatory standards.
Everything in the laboratory—people, instruments, chemicals, testing facilities, methods, documentation—is carefully constructed to produce unbiased test results, the cornerstone of product and patient safety.
A laboratory management system creates an overall framework to guide how every activity should be performed inside the laboratory to protect the integrity of the outcomes it generates.
This article will examine the essential elements of analytical laboratory management and their importance in product quality assurance and implementation.
Laboratory management is a major topic with vast areas of work that cannot be covered in a single piece of writing. However, we have provided references as links where possible.
Table of Contents
Why is effective laboratory management important?
The analytical laboratory in the pharmaceutical industry plays an important role in ensuring that quality products are built into strict specifications and supplied to customers with no questionable safety, purity, or efficacy issues.
That is why regulatory agencies inspect this area properly to ensure plants operate under stringent GLP (Good Laboratory Practice) and GMP (Good Manufacturing Practice) guidelines.
Therefore, managing an analytical laboratory with expert hands is significant to achieving these regulatory and product quality goals.
Analytical laboratory management at a glance
The primary roles of analytical laboratories in the pharmaceutical industry include testing raw materials, in-process materials, and finished products intended for distribution in a marketplace.
Broadly speaking, the test specimens included raw materials, regulatory starting materials, intermediates, active pharmaceutical ingredients (API), in-process materials, drug products, biologics, and medical devices.
A typical laboratory management system includes a variety of activities. The following is a list of comment activities that are not exhaustive.
– Training of laboratory technicians as applicable to the job function.
– Managing hazardous materials.
– Appropriate gowning and personal protective equipment.
– Creating standard operating procedures (SOP), manuals, and instructions to ensure lab members adhere to compliance.
– Preparation of testing method.
– Sampling and management of samples.
– Designing laboratory facilities.
– Handling and storing equipment and related systems.
– Creating a practice for calibration and maintenance of laboratory equipment.
– Controlling and maintaining chemicals and reagents.
– Management of reference standards and volumetric solutions
– Management of microbial cultures,
– Handling culture media and biological indicators.
– Monitoring and controlling the microbiology laboratory environment.
– Document test results in records.
– Method validation, etc.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
GLP training in laboratory management
Qualification, training, and experience of laboratory personnel are priorities in effective laboratory management.
The reliability of product testing depends on the effectiveness of people. Therefore, laboratory personnel must have the right skills, knowledge, and attitudes to perform their work correctly and diligently.
Laboratory management systems must include the types of GLP training required, including induction training, on-the-job skills training, training on SOPs and test methods to understand laboratory procedures, and knowledge of GMP rules.
GLP training must be ongoing.
The company must demonstrate that training has been conducted satisfactorily in the above areas by monitoring each person’s training records.
Additional GLP regulations for personnel include:
– There must be sufficient laboratory staff.
– Personnel must undergo periodic health checks.
– Personnel must wear appropriate gowning.
– Personnel must always follow written procedures.
How do you manage laboratory facilities and equipment?
Laboratory equipment should be maintained clean and stored to prevent contamination.
To meet regulatory compliance, incubators, refrigerators, ovens, and water baths used in the microbiology laboratory should be cleaned and maintained according to an approved schedule.
Laboratory instruments should be calibrated upon installation and according to an approved schedule. They should be used within the calibration period or calibrated upon use.
Laboratory facilities and equipment should be qualified. Automated laboratory equipment and computer-related laboratory systems should include validation of the computerized system.
As a good laboratory management practice, the analytical and microbiology laboratories should be built separately into designated areas with limited access and maintained clean and organized.
Practices should be established at the laboratory to ensure laboratory housekeeping is maintained.
Work surfaces in the microbiology laboratory (e.g., benches, bio-safety cabinets, and unidirectional airflow units) should be cleaned, organized, and disinfected before and after use.
Only those disinfectants that are effective in cleaning the expected types of organisms should be chosen.
Laboratory processes (e.g., sterilization and depyrogenation) should be validated.
The equipment and utilities (e.g., vacuum, compressed air, gases) should be properly designed, commissioned, and qualified.
Laboratory water systems should also be qualified for their intended use. They should be monitored routinely and suitable for the specified test method or test material preparation, adhering to regulatory requirements.
In addition, the water system in the microbiology laboratory must, at a minimum, meet the quality attributes for purified water.
A documented environmental monitoring program must exist for areas with an official air grade. Data must be trended and reviewed regularly, and alert and action limits must be set.
Procedures must exist for cleaning laboratory rooms, benches, and equipment. Only QA-approved cleaning agents are to be used.
All maintenance and repair programs for laboratory facilities and equipment must be documented. Where relevant changes must be managed through the change control system.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Management of laboratory test methods
The management of test methods is another element of analytical laboratory management that helps ensure regulatory compliance.
The laboratory should have a clear policy on developing validated test methods before being deployed for routine use.
Non-compendial test methods used for product release and stability testing should be validated to the established standard when they were developed. Nevertheless, the suitability of all test methods should be qualified under actual conditions of use and documented.
Please assess if the policy covers the following principles while managing laboratory test methods.
1. Source of test methods
Test methods generally originate from one of three sources: official pharmacopeia, new methods from a development laboratory, and/or adopted from literature.
2. Method development
During development, methods are tested for suitability according to the product they are being used for and the analytical validation performance parameters.
3. Published method
Once a quality control method has been suitably developed, it is published in a format that is unambiguous and easy for an analyst to follow. The published method should also reference any instrumentation and corresponding settings.
4. Test method validation
Once a method is published, it must be validated according to ICH Q2(R1) or equivalent criteria. This method validation package should confirm the method’s suitability. The validation report is generally submitted to a regulatory agency to prove the method is valid and ready for use.
5. Transfer of methods to the analytical laboratory
When a test method is suitably validated, it is transferred to the laboratory for commercial use. At this point, QC analysts become responsible for the test method.
6. Use of quality control method in testing
The test method is then used for routine product testing, and if properly validated, the test results should be reliable and robust.
All test methods should be maintained under change control. Other methods, such as in-process test methods, should be validated according to approved procedures.
Personnel performing testing and approving test results should also be trained and qualified.
Waste disposal in laboratory management
Analytical laboratory management in pharmaceuticals should dictate an effective waste management policy to maintain a safe and compliant laboratory environment.
The procedure should include guidelines on waste segregation, proper labeling, storage, and coordination with waste disposal contractors. This will ensure that all waste is managed responsibly and in accordance with legal requirements.
The laboratory waste management procedure should cover the steps from the generation of waste to its disposal.
1. Waste disposal procedure
Laboratory waste disposal and storage should follow the procedure set by the lab manager.
You can use labeled plastic drums to dispose of laboratory waste.
Store these drums in waste cupboards until disposed of, with spare drums in the laboratory storage area.
Keep labels for the drums in the waste cupboard. Remove existing labels on the drums or deface them before use.
2. Sorting and disposing of waste
When laboratory waste is created, dispose of it into a waste drum using a designated funnel, ensuring adherence to lab management practices.
Waste is then sorted by chemical categories into larger drums, as instructed by the lab manager. A chemical waste disposal form can be used for recording purposes.
Laboratory staff must remove the waste drum when it is full or turns smelly, place it on a trolley, and move it carefully to the disposal area.
3. Use of safety cabinets and logbook
Place the waste drums in yellow safety cabinets, with one cabinet for non-flammable waste and another for flammable waste.
Keep one “hazardous waste logbook” in the non-flammable waste cabinet, recording the date and type of waste deposited.
Use an approved contractor to remove the waste weekly or earlier if quantities exceed legal limits.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Sampling in laboratory management
The sampling process, sample handling, and testing must be performed to comply with quality standards and are essential parts of effective laboratory management.
You must have standard operating procedures and instruction manuals in place to describe sample processing workflow, including:
– Sample receipt
– Proper sample labeling and sample log-in
– Prevention of sample loss
– Sample mix-ups and contamination
– Verification that information on the sample label matches the accompanying document submission
– Identification of potent
– Toxic or hazardous compounds requiring special precautionary measures
– Sample storage at room temperature unless otherwise indicated
– Tests performed within a defined period after sample receipt
– Investigation of sample mix-ups or contamination.
– Sample retention will be maintained until all tests are completed and a determination is made as to whether a result is valid or invalid.
sample destruction or disposal.
– Controls for adherence to compliance cross-contamination should be available and implemented.
Analytical laboratories in the pharmaceutical industry follow a series of documented steps to test a sample.
You need validated test methods, qualified instruments, reference standards, and approved specifications to support each step.
1. Received labeled sample: The analytical laboratory maintains a sample receiving register. This register logs the batch and sample number, the number of samples provided, and the time and date the sample first entered the laboratory.
2. Prepare sample and set up: Many quality control methods require sample preparation before running the test. The test method should describe exactly how the sample is prepared.
3. Run the test sample: Once the sample is prepared, it is included for running the test.
4. Verify system suitability: For instrumental runs such as HPLC/GC, the test method usually includes a verification that the entire instrumental system is performing satisfactorily on the day of the run. This is called “system suitability testing” (SST).
5. Calculate/check result: Once the sample has been run, either the instrument or the analyst will calculate to arrive at a result. It is essential to double-check all calculations before finalizing sample results.
6. Out of specifications (OOS)? Analytical laboratories have written procedures for handling and investigating only results that appear to be OOS.
7. Current test method: Before commencing any assay, the analyst should refer to the current version of the test method. The test method should be available at the worksite.
8. Qualified instrument: Samples must be processed through qualified and calibrated instruments.
9. Reference standard: Many tests require comparing the sample to an official reference standard to meet regulatory requirements.
10. Approved specification: Analytical laboratories maintain published and approved specifications for each starting material and finished product. When calculating and checking results, the analyst should refer to the current specification to decide the sample’s status. These specifications are often registered with the regulatory agency and cannot be changed.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Handling of reagents and reference standards in the laboratory
Safe and effective handling of chemicals, reagents, and reference standards is vital in analytical laboratory management.
Laboratory chemicals that are used for testing, such as reagents, buffers, reference standards, microbial cultures, microbiological indicators, and culture media, should only be used for their intended purposes.
Some laboratory reagents are classified as dangerous goods, such as explosives and gase, active materials, e.g., cytotoxics, flammable materials, peroxides and oxidizing agents, poisons and infectious substances, radioactive materials, and corrosives.
There must be a process of safeguarding these chemicals and using them only in the approved quantity. After use, they should be kept in a safe and secure location inside the laboratory.
Reagents
Proper labeling and tracking are key when it comes to reagents. Every reagent, whether purchased or prepared in the laboratory, needs a clear label that includes its name, batch or lot number, and vendor details.
For those made in-house, additional information is necessary to identify them uniquely. If reagents are subdivided, labels should allow us to trace back to the original source.
Recording the receipt date and the first opening date is crucial and should be done on the label or associated documentation.
Maintaining records according to SOPs and regulatory requirements ensures all reagents and chemicals in the laboratory are traceable and accounted for.
Another vital aspect is the Storage of reagents. Reagents must be stored according to their specific requirements, ensuring they remain effective until expiration.
If the usage is to be extended beyond this date, proper documentation and approval are necessary.
Expired materials should be discarded promptly. An investigation should be conducted if expired materials are used in error.
Reference Standards
Reference standards used for testing production materials, actives, drug products, and medical devices should be obtained or established, used, controlled, maintained, and approved according to written procedures approved by the site quality assurance.
Labels must provide all necessary details to uniquely identify reference standards used in analytical testing, including the material’s name, batch number, and vendor information.
Like reagents, subdivided reference standards should be traceable back to their source.
These standards must be linked to their documentation, ensuring traceability. Just like reagents, the receipt date and first opening date should be recorded, either on the label or in related documentation.
Storage conditions must follow specific guidelines to maintain the reference standards’ integrity.
A system should be in place to inform users about the status of reference standards, including any changes or discontinuations.
In both cases, maintaining a clear, accurate record and adhering to labeling, storage, and documentation protocols is essential for ensuring the integrity and traceability of reagents and reference standards in the laboratory.
Material Safety Data Sheet (MSDS)
An MSDS provided by the supplier is designed to provide both workers and emergency personnel with the proper procedures for handling or working with a particular substance or when an accident occurs.
The MSDS includes information such as:
– Physical data on safe handling
– Toxicity limits
– Health effects on exposure
– First aid on exposure
– Reactivity with other chemicals
– Safe storage
– Sole disposal
– Use of personnel protective equipment
– Recommended spillage and leakage procedures
Documentation and records in laboratory management
Effective documentation and record-keeping are two of the most important parameters of effective laboratory management.
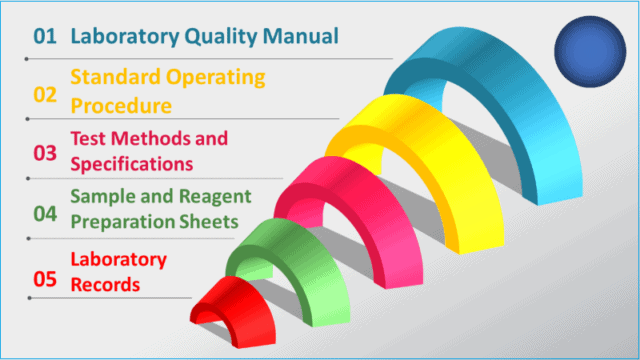
When it comes to laboratory documentation, there is a hierarchy of documents to manage.
First, develop quality manuals and policies that provide a high-level overview of all laboratory activities, governance, and regulatory compliance.
Secondly, standard operating procedures, manuals, and test methods should be established to guide how to perform certain activities.
Finally, managing the laboratory records provides evidence of what was actually done.
When designing a laboratory documentation management system, please follow the hierarchy step by step.
1. Laboratory quality manual and policies
The laboratory quality manual and policies are top-level documents that describe the laboratory’s overall management and organization. They should reflect the requirements under GMP rules.
2. Standard operating procedures
Standard operating procedures (SOPs) provide more detailed and specific requirements for each laboratory quality element.
For example, SOPs describe how to handle a sample, conduct an audit, release a result from the laboratory, and manage a complaint. SOPs are generally not specific to test methods.
3. Test methods and specifications
Test methods provide specific stepwise directions on how to execute a test procedure in a standardized manner properly.
A test method is specific to analysis and instrumental techniques such as High-Pressure Liquid Chromatography (HPLC).
Specifications generally accompany test methods and provide pass/fail criteria and acceptance criteria for a test method, such as system suitability or control limits.
4. Sample and reagent preparation sheet
Sample and reagent preparation sheets are useful for preparing laboratory solutions, standards, and working reagents.
Accurate instructions and records of these preparations are important in a laboratory. Usually, these sheets are linked to specific test methods. Equally important are the instructions for calibrating standard solutions.
5. Laboratory records
Testing records include laboratory analyst notebooks, specific testing sheets, analytical printouts, electronic records such as chromatographs, and ancillary records that support the laboratory’s compliance. Ancillary records would include calibration reports, training records, and environmental monitoring.
For example, laboratory records should be maintained for each sterilization load with the evidence of:
– Date and time of sterilization
– Identification of the person performing sterilization activities
– Identification of all equipment and components in the load
– Cycle identification
– Batch number or run number
– Review of sterilization cycles, including indication of pass/fail status,
– Retention of sterilization records according to retention policy.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Auditing practice in laboratory management
Good internal auditing practice calls for a collaborative focus on “improvement” in laboratory management.
Auditing a testing laboratory guarantees that the laboratory satisfies all compliance requirements, which benefits everyone.
Any laboratory using a laboratory quality management system must conduct audits as part of a quality assurance program. ISO-17025 and regulatory bodies require this.
Laboratory managers and supervisors are responsible for developing audit cadence.
Laboratory managers routinely perform audits to confirm that best practices and procedures are followed in the laboratory, spot issues, and identify areas for improvement.
Consider the following when developing the scope of laboratory audits:
– GLP compliance audits
– Regular monitoring of test performances
– Quality system reviews
The credibility of the laboratory hinges on a good laboratory management system.
Documentation and records maintained in the laboratory should be able to withstand auditing. Specifically, internal audits aim to verify that:
– Results are reliable and accurate
– The information flow to customers is timely
– Test results are unambiguous
– The laboratory is economically efficient
– Historical records and data are retrievable
– The laboratory operates independently in quality assessment.
Effective laboratory management dictates internal audits to be conducted in the following areas:
– Safety studies
– Compliance
– Regulatory
– Investigation
– Trend Analysis
Auditors will likely want to see laboratory notebooks, test methods, and “objective evidence” on how you conducted a test or calculated a result.
Have a list of all the documentation that might be needed and ensure they are readily available.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Conclusion
Effective laboratory management in the pharmaceutical industry ensures regulatory compliance, product quality, and patient safety. Without the proper management, the integrity of laboratory test results will be questionable.
Analytical laboratories in the pharmaceutical industry are tightly regulated environments.
Successful laboratory management involves a comprehensive system that ensures all activities within the lab are conducted to maintain the integrity of the outcomes, which is critical for product quality assurance and regulatory compliance.
Laboratories frequently use chemicals, reagents, and reference standards. Proper labeling and tracking of reagents are fundamental.
Every reagent, whether purchased or prepared on-site, must be clearly labeled with essential details like name, batch number, and vendor information.
Effective storage practices are crucial to maintaining reagent efficacy until their expiration date, and proper documentation is required for any extensions.
Reference standards are used as the benchmark of every test and must be meticulously managed. Their use must be traceable through their documentation and records.
A system should be in place to inform users about the status and any changes regarding reference standards.
Accurate documentation and record-keeping are the backbone of laboratory management. This includes quality manuals, standard operating procedures (SOPs), test methods, and preparation sheets.
Ensuring these documents are well-maintained and readily available is critical for compliance and auditing purposes.
Regular internal audits ensure that laboratory practices adhere to regulatory standards and GLP guidelines.
Audits help identify areas for improvement and confirm that the laboratory’s operations are efficient, reliable, and economically viable.
They also ensure that test results are accurate and that historical data is retrievable.
Laboratory personnel’s competency is a must for reliable testing. Ongoing training programs covering GLP, GMP, SOPs, and specific test methods ensure that staff remain skilled and knowledgeable.
Proper training records must be maintained to demonstrate compliance with training requirements.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.