What is analytical method validation in GMP
- Kazi
- Last modified: April 2, 2024
What is analytical method validation?
FDA – Process Validation Guidelines (1987) defines “Analytical Method Validation as the process of establishing documented evidence which provides a high degree of assurance that a specific process such as analytical test method, will consistently produce a product supported by assay results meeting its predetermined specifications and quality attributes (i.e. accuracy, precision, etc.)”
To clearly understand analytical method validation, you need to define what is a test method and what is validation.
According to Guidance for Industry Bioanalytical Method Validation 2001- CDER “a method is a comprehensive description of all procedures used in sample analysis”.
Conversely, validation can be defined as a documented program that provides a high degree of assurance that a specific process, method, or system will consistently produce a result meeting predetermined acceptance criteria.
Validation is proving that any procedure, process, equipment, material, activity, or system actually leads to the expected results per the principles of Good Manufacturing Practice.
Within this context, you also need to have familiarity with the term “Specification”.
Specification means a list of acceptance criteria such as numerical limits, ranges, or other criteria for the test method described.
It establishes the set of criteria to which a material should conform to be considered acceptable for its intended use.
Conformance to specification means that the material will meet the listed acceptance criteria when tested according to the listed analytical procedures.
Specifications usually relate to starting materials, components, bulk products, and finished products but may also apply to critical steps of manufacture where failure to meet the acceptance criteria would result in defective products.
Analytical method validation guarantees that your test method is robust enough to provide evidence if your product meets the predetermined product specification or conforms to the failure of a product.
What is test method validation?
Test method validation generally means the same as analytical method validation where the terms are interchangeable.
Like analytical methods, test method validation is the process by which it is established that the performance characteristics of the test method, such as precision, accuracy, specificity, linearity, Limit of Detection (LOD), Limit of Quantitation (LOQ), and robustness meet the requirements for the intended applications.
Why is analytical validation needed?
If you are in a business to manufacture and sell medicinal products or medical devices for human use your therapeutic goods must have to be registered with your local regulatory authority with sufficient evidence that your product is safe, pure, effective, and traceable.
It is a GMP regulatory requirement to produce evidence-based determination that the analytical methods you have employed to analyze your products are validated. Meaning, that the analytical methods consistently generate true results with precision and accuracy each time every time. A planned analytical method validation process can generate a high level of confidence for your products.
What are the prerequisites for analytical method validation?
In order to validate your test method, there are some important prerequisites that you have to consider before the validation process begins. Those are:
– Your laboratory instruments are qualified
– You have a well-developed documentation process
– Your reference standards are reliable, stable
– You have trained and qualified analysts
– Be aware that the test method is robust to factors that may change or be uncontrolled
– You have a well-documented method validation protocol and reporting system
What type of analytical procedures require validation?
You need to identify those test methods that are used to determine your product’s quality attributes and efficacy of the processes that directly or indirectly impact the quality of your products. Then include those analytic methods for the validation regime. For example:
– Test methods for the identity of active pharmaceutical ingredients (API)
– Qualitative measurements for impurities/degradants
– Limit tests for impurities/degradants
– Quantitative assays of an API or drug product active material
– Physical product characteristics, e.g. dissolution rate
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
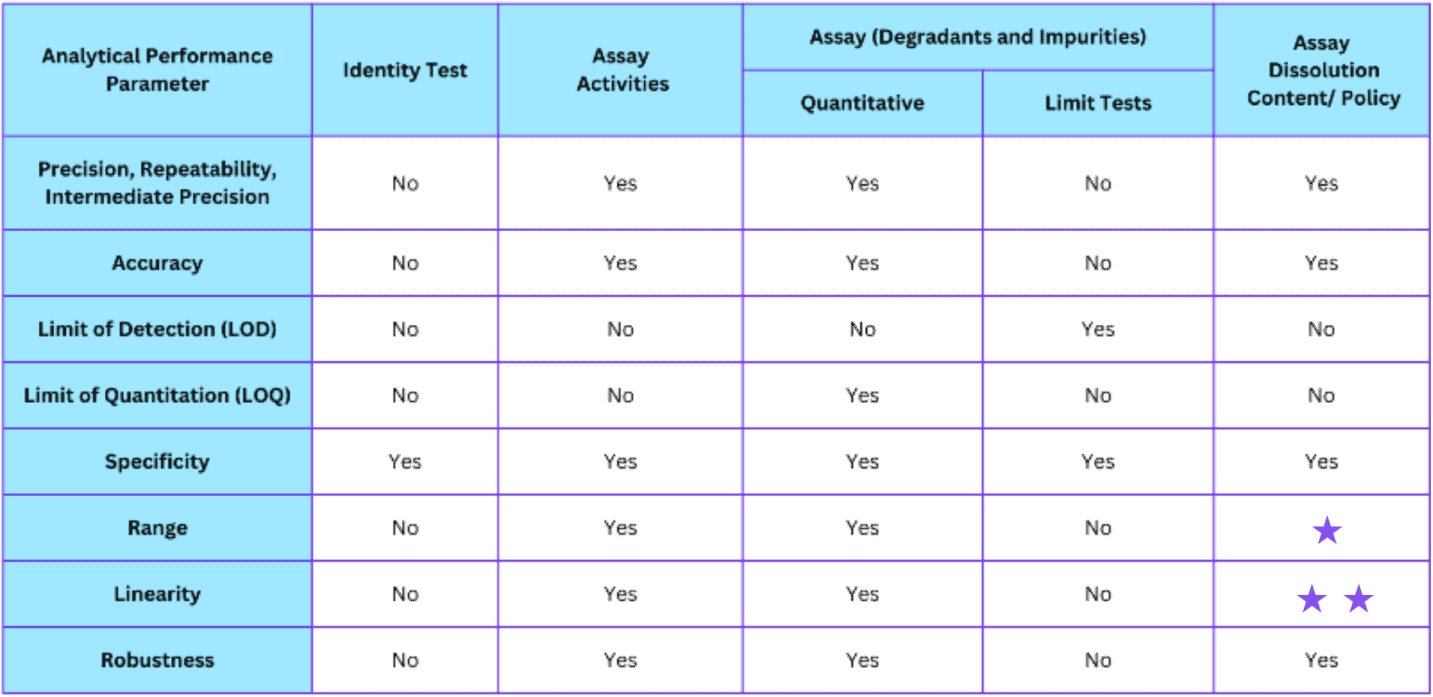
Performance Parameters Required for Assay Validation
(Ref. USP 25 <1225> and ICH Q2A/B)
* may be required depending on the nature of the specific test
* * lack of specificity in a test may be compensated for with additional tests
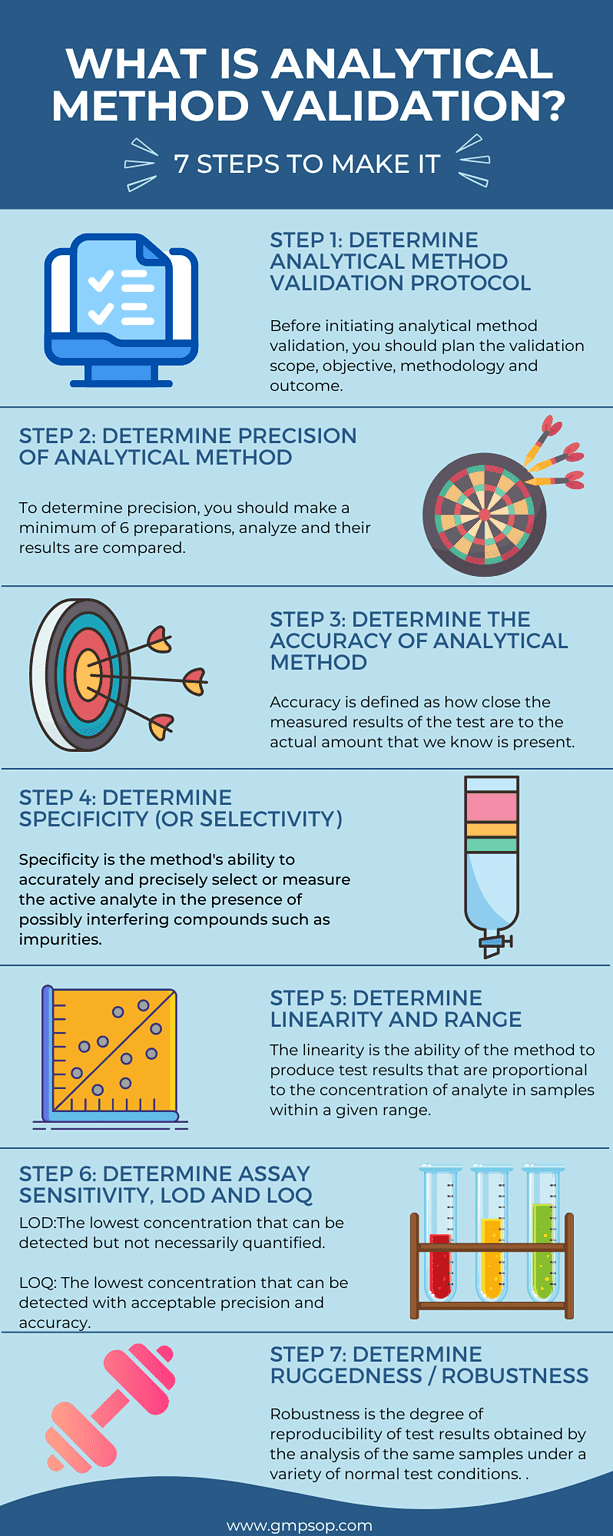
What are the steps of analytical method validation?
To successfully complete an analytical method validation, you will need to validate all or a combination of the following parameters:
– Method precision
– Method accuracy
– Method selectivity/specificity and system suitability
– Method linearity and range
– Method sensitivity
– Method ruggedness and robustness
Following are some fundamental steps that are taken during analytical method validation. However, different validation projects require unique steps to follow depending on the type of test method under validation, laboratory conditions, equipment qualification, criteria of sample to be tested, reference standard and controls to be used source of the analytical method, etc.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Step 1: Determine items to include in the analytical method validation protocol template.
Before initiating analytical method validation, you should plan the validation scope, objective, methodology, and outcome. To that purpose, you need to create a validation protocol. The protocol will generate the blueprint of all activities to be undertaken during the validation approach and results are documented and reported. Typical items that constitute a method validation protocol are:
– Statement of protocol scope
– Responsibilities for approval, execution and final review
– List of required materials and instruments
– Statement of the test method (in final draft)
– Details of the experimental design for verifying each performance parameter
– Documents and forms for recording validation results
– Acceptance criteria for each performance parameter
Step 2: Determine the precision of the analytical method
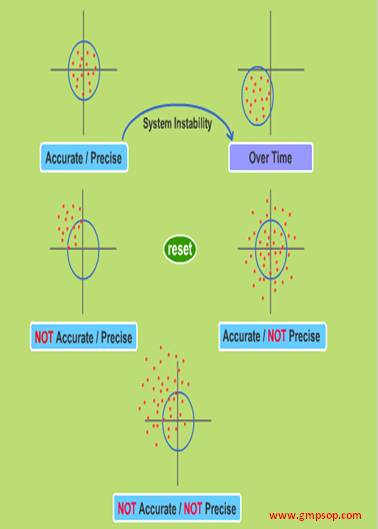
Precision is defined as the closeness of results with respect to each other. To determine precision, you should make a minimum of 6 preparations, and analyze and their results are compared.
The precision of a test method is measured in three ways:
– Repeatability: measures the precision of the operating system.
– Intermediate precision: measures the precision of the analytical method.
– Reproducibility: measures the precision between different analysts on different days and using different equipment.
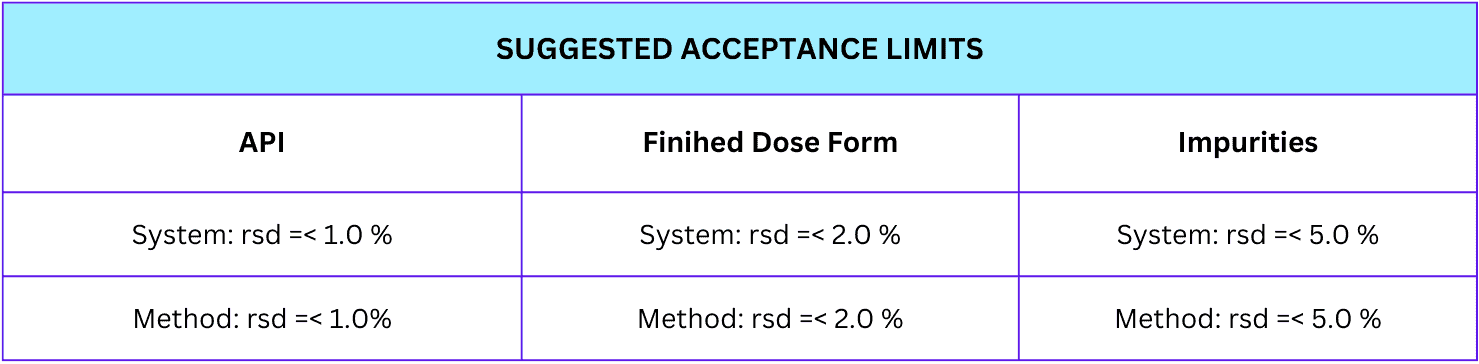
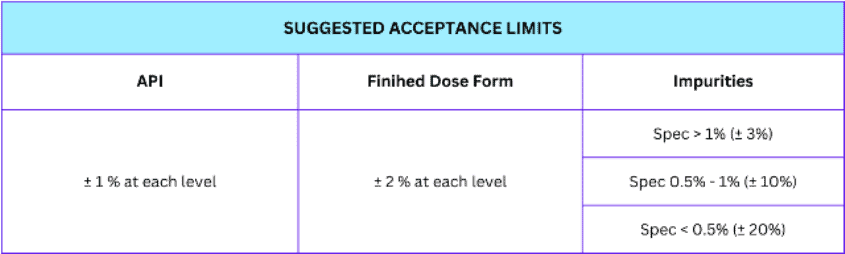
We accept precision according to pre-defined criteria that are relative to the method itself and the product specification. Ideally, the repeatability of chromatographic methods should be < 1.0%.
There are suggested precision acceptance limits depending on the type of analytical samples to be analyzed. Refer to the table on how the acceptance limit is categorized.
Step 3: Determine the accuracy of the analytical method
Accuracy is defined as how close the measured results of the test are to the actual amount that we know is present.
There are a few ways to determine test method accuracy:
– Spike the active into a placebo matrix using amounts ranging from 25% to 150% of dose strength.
– Standard addition of active to the drug matrix.
– Direct comparison of two alternate methods for equivalence.
We should also consider the prepared sample’s stability. If samples degrade, this may affect our accuracy. This check applies to standards and samples standing for a minimum of 24 hours on the bench.
How to calculate accuracy?
% Accuracy = 100 x [(Experimental amount – Theoretical amount)/Theoretical amount]
Accuracy may also be expressed as “bias” of the method, e.g. -1.2% bias.
There are suggested accuracy acceptance limits depending on the type of analytical samples to be analyzed. Refer to the table on how the acceptance limit for accuracy is categorized.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
What is method capability?
Capability is a measure of the method’s ability to consistently produce results that are within specification. The capability is dependent on:
– The specification width
– The accuracy of the method
– The precision of the method
How to calculate method capability?
Cp method = [ (USL – LSL) – 2 x | average bias | ] / 6 x σ method
Cp > 2.5 Excellent capability
Cp 2.4 to 1.0 Good to poor capability
Cp < 1.0 Unacceptable
Keys:
USL = Upper specification limit
LSL = Lower specification limit
bias = Accuracy
σ method = Intermediate precision
Step 4: Determine the specificity (or selectivity) of the analytical method
Specificity is the method’s ability to accurately and precisely select or measure the active analyte in the presence of possibly interfering compounds such as impurities, degradants, excipients, solvents, and the drug matrix/excipients.
For HPLC (High-performance liquid chromatography) or GC (Gas chromatography) assays, the ability of the method to separate interfering compounds is an indication of specificity.
Verification of specificity is important for methods used for stability-indicating assays.
How to check the specificity of analytical method?
Add the analyte to each of the potential interfering compounds and assess its ability to meet the following:
– Ideally, no peaks are present in the chromatogram of the mobile phase or diluents. If peaks are present, they elute with the solvent front.
– If peaks are present, they should elute at RRTs (Relative Retention Time) that are not the same or similar to those of the API, internal standard, or any impurity peaks.
– If any other peaks are present, they should also elute at RRTs that are not similar to those of any other peaks in the chromatogram.
– All excipient peaks from both the fresh and degraded workups should elute at RRTs that are not the same or similar to those of the API, internal standard, or impurity peaks.
What is system suitability for HPLC methods?
Applicable generally for chromatographic test methods, system suitability is the determination of instrument performance (e.g., sensitivity and chromatographic retention) by analysis of a reference standard prior to running the analytical batch.
Follow the link to learn more about System Suitability for HPLC analysis.
Step 5: Determine the linearity and range of the analytical method
The linearity of an analytical method may be defined as the ability of the method to produce test results that are proportional to the concentration of analyte in samples within a given range.
The range of a method is the interval between upper and lower concentrations that have been demonstrated to be accurate, precise, and linear by method validation studies.
You should test at least 5 samples of differing concentrations in triplicate, (i.e. injected three times) to establish the linearity of the response and the concentration range over which this linear response applies.
The five concentrations are made up by:
– Accurately weighing sample then transferring it into volumetric glassware, OR
– Making appropriate dilutions from an accurately prepared stock solution
Linear regression analysis is then applied to the results to demonstrate the linearity of the method over the range of concentrations studied. The range of concentrations studied is usually 50% ‑ 125% of the target concentration of the active in the sample. Degradant concentrations are evaluated for each particular method.
The correlation coefficient, r, should be > 0.99 for the range selected. This is the range where the slope is linear.
The method should have a linear response over at least 75-125% of the target concentration of the analyte.
Step 6: Determine Assay sensitivity, Limit of Detection (LOD) and Limit of Quantitation (LOQ) of the analytical method.
Sensitivity is the response obtained for a given amount of analyte. There are two factors that are used to verify sensitivity:
The limit of detection (LOD): The lowest concentration that can be detected but not necessarily quantified. LOD is a parameter of limit tests.
The limit of quantitation (LOQ): The lowest concentration that can be detected with acceptable precision and accuracy. A parameter of quantitative impurity assays.
What are the ways to determine LOD and LOQ?
– Evaluation of acceptable detection for LOD or accuracy and precision at the nominated LOQ.
– Evaluation of signal-to-noise ratio for instrumental methods that exhibit baseline signals.
– Precision of response and the slope where:
LOQ = (10xσ)/b and LOD=(3.3xσ)/b
σ = standard deviation of the response
b = slope of the calibration curve
There are no specific acceptance criteria for LOD and LOQ.
The general validation should aim for active APIs:
– LOQ should not be more than 0.5%, and preferably much less
– LOD should not be more than 0.25%, and preferably much less
– Precision should be 5-10% at the defined limit for LOQ
– LOD signal-to-noise ratio should be 3:1 at the defined limit
– LOQ signal-to-noise ratio should be 10:1 at the defined limit
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Step 7: Determine the Ruggedness and Robustness of the analytical method
Ruggedness/Robustness is defined as the degree of reproducibility of test results obtained by the analysis of the same samples under a variety of normal test conditions. Ruggedness refers to external factors that can affect the method. While robustness refers to internal factors that can affect the method.
Ruggedness or robustness can be determined by measuring the accuracy and precision under a variety of conditions including:
– Different days
– Different analysts
– Different laboratories
– Different instruments
– Different columns
– Different reagent lots
– Different assay temperatures
– Different elapsed sample preparation/assay times
If ruggedness studies indicate that any factor is likely to influence the assay, then this should be controlled by the published test method.
Is analytical method validation necessary for the transfer of test method?
Analytical method validation is strongly recommended during the transfer of test methods. Analytical Method validation ensures that a method is robust enough to transfer to another laboratory, e.g. from the R&D to a Quality Control laboratory. The validation generates recorded evidence that the transferred methods behave and analyze exactly in the same parameters to achieve true results in a changed condition.
Method transfer requires documented evidence (a protocol and procedure) that should verify the following attributes of the method:
– Precision
– Accuracy
– Linearity/range
– Ruggedness/robustness
The transfer protocol qualifies the new laboratory, trains its staff, and ensures that the new site can perform the method to the same standards as the originating site.
What are the requirements for analytical method transfer?
You are expected to produce the following documentation during a method transfer:
Detailed method: The procedure should be unambiguous with example chromatograms of the standard, a typical sample, and a system suitability limit with calculations.
Method development report: The report reviews the method development and provides justification for the choice of operational parameters and conditions.
Transfer protocol: The protocol details requirements, timing, responsibilities, acceptance criteria, etc.
Validation report: The summary should include a summary of validation activities and results.
What are the requirements of stability-indicating assays?
The validation of stability-indicating assays needs to verify the stability-indicating nature of the method.
There should be a defined degradation pathway for the active.
Stability indicating method should:
– Be selective for degradants
– Be robust to interference by-product matrices
– Be able to chromatographically separate retention times
– Mass-balance account for >90% of contents
Method validation of compendial (pharmacopoeial) tests
Compendial methods are those that come directly from pharmacopoeias i.e. EP, USP. They are generally considered reliable under the usual conditions of use.
You should validate Pharmacopoeial methods for the use of finished medicinal goods.
Every laboratory has different conditions and equipment and may apply the method in different ways to different products. Therefore, you cannot assume that a compendial method is validated. You need to verify its suitability under the “actual conditions of use” in your laboratory.
Conclusion
Reliable analytical method validation is a fundamental Good Laboratory Practice (GLP) requirement. It is also important for product registration with regulatory authority and during GMP inspection of laboratories.
Analytical method validation generates evidence that your test methods are robust and reproducible to ensure your product is safe, pure, effective, and traceable for human use. You have to plan your method validation approach in a protocol, develop specifications, undertake validation steps sequentially, and report the outcome.
You should give further considerations during analytical method validation such as:
– List performance parameters required and their acceptance criteria.
– Identify what is involved in an analytical method validation protocol.
– List requirements for an inter-laboratory method transfer.
– Understand the requirements of stability indicating assays.
– Understand why compendial methods need to be validated.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.

One thought on “What is analytical method validation in GMP”