
How to Prepare SOP for Annual Product Quality Review in GMP
- Kazi
- Last modified: September 17, 2024
SOP for annual product quality review is intended to provide information of a pharmaceutical product performance. You should perform product quality review annually or periodically to determine if any adjustments to product specifications, manufacturing process or raw material supply are required to further improve the product.
You should develop a standard operating procedure (SOP) and relevant templates that will describe how to conduct annual product quality review through analysis of individual aspects of product quality. Analysis, reporting and tending of your product quality should be done on templates to maintain consistency with future reviews.
If you are looking for guidance on how to prepare a SOP following is a step by step process to do that. The SOP should be used as a guidance for performing and documenting annual product quality reviews.
What are the scope of product quality review SOP?
Annual product quality review helps evaluate the quality of the product by reviewing all the deviation investigation, any changes in the process, validation, recalls, customer complaints and if any change in specification.
In general, the product quality review SOP should have the guidance on following review areas but not limited to:
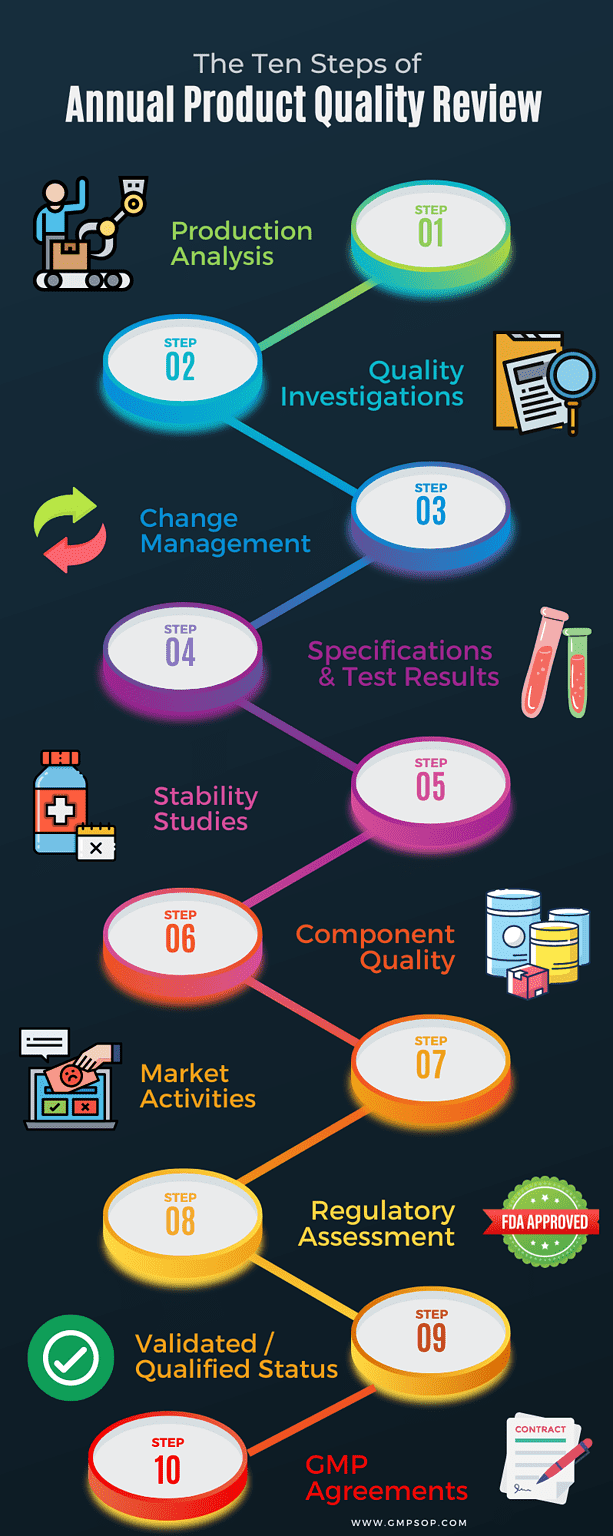
– An analysis of the production process
– A review and investigation of all deviations and non-conformances
– A review of all approved change controls that could impact product quality, validation status or regulatory filing for the product
– An analysis of the microbiological and chemical test results and conformance with specifications
– An analysis of stability studies and associated test results
– An assessment of component quality based on a review of the starting materials and primary packaging that comes into direct contact with the product
– A review of market activity, concentrating on product returns, complaints and recalls
– A regulatory assessment consisting of any changes to the specifications as registered by all relevant regulatory agencies
– An assessment of the validated or qualified status of all processing, cleaning, analytical methods, automated controls or packaging validation
– An assessment of relevant suppliers and currency of relevant GMP agreements
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Who are responsible for conducting annual product quality review?
A. Quality Assurance Department: Quality assurance department is responsible for the reviewing and compiling all the deviation, recalls and customer complaints.
B. Quality Control Department: Review specification to make sure it is current and review stability data to confirm expiration date.
C. Validation Department: Review process validation and determine if additional validation is required for process, equipment or method.
D. Regulatory Department: Regulatory department is responsible for providing the information on any regulatory filling for the product. Regulatory department is responsible for evaluating any specification or process change which may impact regulatory filing requirements.
E. Manufacturing Department: Manufacturing department is responsible for the complete review of manufacturing process to determine if there were any changes made to the process during the year.
Which reference documents you should utilise while preparing your SOP?
Following are some regulatory requirements that you would like to follow while developing your annual product quality review program.
21CFR 180 (e) Subpart J – Records and Reports
The ten steps of annual product quality review
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
What should be included in product quality review SOP?
Following are some steps and business rules you can follow while developing your standard operating procedure on annual product quality review process.
A. Annual product quality review should be completed on annual basis for each product manufactured by your company.
B. Annual product quality review will contain information about all the batches of products manufactured during that period. If you have similar product range, you may group those products into a family with similar formulation and properties.
C. Include a segment for trend review of manufacturing process and finished product test data generated for number of batches manufactured during that period.
D. Include a section for stability data review to collect evidence of chemical, microbiological, and physical degradation and investigations concerning data discrepancies. You should be able to verify that the data confirms the registered expiration date. Also any stability trend or deviation noticed during review should be detailed in the report.
E. A summary of all the product returned due to quality issue should be included in the annual product quality review SOP. A review of market activity should include all quality-related product returns, complaints and recalls.
F. The SOP should include details on complaint investigations and recommended corrective and preventive action list. Review involves determination if the customer complaints received represents a significant increase or decrease from the previous year. If any corrective action required due to increase in the complaints should be reported in the annual product review. A summary of all complaints or adverse events during the review period is required. Details of how each issue was resolved should be provided.
G. The retention samples based on statistical sampling procedure from each product should be visually inspected and recorded in the annual product quality review report.
H. Validation department should report any validation performed on process, equipment or method should be included in the annual product quality review report. All Validation / Qualification studies either commenced or completed in the review period must be assessed. These may include:
– Packaging validation
– Cleaning validation
– Analytical method validation
– Equipment validation
I. Any significant issues and deviation that were identified must be discussed and a resolution provided. The effectiveness of the validation must be determined.
J. Regulatory department should provide information on regulatory submission due to change in the process or specification. A summary of changes, in terms of specification registered with local or international government regulators must be included. Any post marketing commitments should be reviewed and status assessed.
K. The SOP should cover review of all the deviation investigation report to evaluate impact on the quality of the product. Quality assurance department should compile a list of all the deviation and out of specifications investigations.
L. The review summary should contain information on corrective and preventive actions, recommendation that resulted from the review. Quality assurances department is responsible for compiling all corrective actions and implement fixes.
M. A conclusion will be formulated based on the overall findings from the annual review and appropriate corrective action and preventive action will be recommended.
N. Annual product quality review report should be approved by QA, QC, Regulatory affairs, Manufacturing and Validation department.
All the annual product review reports should be reviewed by the senior management for the quality of the product.
How to document summary report on annual product quality review
The SOP for annual product quality review should details how to collect, analyse and report data and information to provide a complete picture on product quality, any departure from expectations and corrective actions to be taken to prevent the degradation. The summary report of annual product quality review should provide a comprehensive overview of the data contained within the different elements of the product review.
The summary needs to provide:
– An analysis of the products performance
– A review of all the elements reported
– A summary of any trends or concerns
– Identification of any previous issues or corrective actions
– A conclusion as to suitability of the product for on-going production
– A rating of product performance based on the report
The summary should stand alone as a report and provide sufficient detail for a senior management to make an assessment on product performance.
Conclusion
The main objective to develop an SOP for annual product quality review is to provide a consistent approach on product quality review process.
You should have standard operating procedure and templates that will describe how to review individual aspects of product quality and tending of your product quality.
The review analysis should cover areas such as production process, product specifications, major deviations, out of specification results, validation status, test methods and test results, stability studies, change of raw materials and packaging components, complaints, recalls and other market and regulatory actions.
You must prepare a comprehensive summary report of your findings and observations derived from the review. Use statistical analysis to prove if the product quality is maintaining its expected quality. If you discover any adverse trend during the review process take necessary corrective and preventive actions. Prepare your recommendations to improve the product quality in your summary report.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.

I apologise, but, in my opinion, you are not right. I am assured. I can prove it. Write to me in PM.