What is environmental monitoring in pharmaceutical industry
- Kazi
- Last modified: March 15, 2025
Environmental monitoring in pharmaceutical industry is a documented program that describes a plan to monitor particulates inside the processing and manufacturing areas routinely. The program includes a corrective action plan when action levels are exceeded.
An environmental monitoring program provides meaningful information on the quality of the aseptic processing environment when a given batch is being manufactured and on environmental trends in the manufacturing area.
When you establish an efficient program it can identify the potential routes of contamination, allowing for implementation of corrections before contamination occurs.
Table of Contents
What is environmental monitoring in pharmaceutical industry?
Environmental monitoring in pharmaceutical industry provides information on the non-viable particulate and microbiological (viable) quality of the pharmaceutical environment being monitored.
Through environmental monitoring, you can gain insight into the presence of contaminants in the environment where pharmaceutical products are processed. You can also check the effectiveness of common processing steps such as cleaning, housekeeping, gowning, air handling, etc. to ensure that contaminants do not compromise the safety of your products.
Some pharmaceutical products, such as injectables and vaccines, are prepared in aseptic environments, which are also called cleanrooms and controlled environments. Processing areas are classified differently and require differential levels of monitoring.
You must monitor controlled environments prescribed by local regulatory guidelines and pharmaceutical industry practices.
You have to develop plans, policies, protocols, and equipment to monitor your controlled environments appropriate for using that environment for pharmaceutical production. Environmental monitoring programs must be based on the area’s processing requirements and risk assessment.
Even though environmental monitoring is most closely associated with aseptic processing, it should include monitoring all environments at different levels where products and their components are manufactured, especially where there is a risk of product contamination.
The practice will help you maintain awareness of the microbiological environmental conditions during manufacturing activities.
pharmaceutical processing areas that require environmental monitoring?
During environmental monitoring, you must focus on air, surfaces, and people, which frequently come into contact with products. In addition, you should monitor all water and gases used in product manufacturing and feed water to the final water purification system (WFI System).
You should develop room sampling plans in your monitoring practice to define the type of testing, alert and action levels, frequency, and location of test samples to collect for air, surface, and personnel monitoring.
Room sampling plans should consider the configuration of machinery and equipment in the room, airflow patterns (based on validation studies), and personnel and material flow.
You should prepare rationale statements for room testing and for determining alert and/or action levels. Each environmental monitoring program is unique to the product, process, and plant.
What are the benefits of environmental monitoring?
If managed correctly, a robust environmental monitoring program will usually provide early notice of a breakdown in control measures.
Monitoring is, however, a passive reading of the environment. It cannot be used to justify bad practices with supposedly good data. In such a case, all that is indicated is that the environmental program is also poor.
To sum it up, the environmental monitoring program:
– Provides you with crucial information on the quality of the processing environment during manufacturing.
– Helps you to aid the release decision and determine if a batch could potentially be contaminated.
– Helps your firm to react to changes in the environment thus allowing better control of the environment, which in turn will make the likelihood of contamination less
It is of utmost importance to have a clear and well-defined environmental monitoring policy on when and at what level to identify microorganisms.
This article will instill confidence in your actions and ensure the effectiveness of your environmental monitoring program.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
What are other factors to consider during monitoring?
During the environmental monitoring plan, in addition to monitoring air, surfaces, and people, you should assess factors that can potentially contribute to the product’s contamination.
You should implement ongoing monitoring of the factors such as:
– Temperature
– Humidity
– Pressure differentials
– Air within the classified and critical areas
– Utilities that may contact open product or components
– Equipment and critical surfaces within a classified or critical area
– Sanitization
– Operating personnel
What is an environmental monitoring plan?
Establish what to look for when designing an environmental monitoring plan, e.g., common aerobic bacteria, molds, and/or anaerobic bacteria.
It is equally important that you set the objectives of the environmental monitoring plan.
Normally, the plan should provide supporting data to demonstrate the adequacy/performance of the contamination/environmental control measures taken.
The environmental monitoring plan should include:
– People who will be working in different zones
– Monitoring people while exiting the area
– Monitoring aseptic practice e.g., filling
– Your controlled processing areas
– Monitoring airborne contamination
– Surface monitoring
– Materials you will be using
– Components that are in the controlled areas
– Pre-filtration/pre-sterilization bioburden
You can use techniques such as Hazard Analysis and Critical Control Point (HACCP) to focus a monitoring plan on the areas where the product is at greatest risk of contamination.
Product as well as process characteristics should be taken into account, such as:
Product characteristics:
– Terminal sterilization versus Aseptic Processing
– Microbiological vulnerability
Process characteristics:
– Process design
– Product flow
– Personnel flow and numbers
– Working patterns (shifts?)
– Plant occupancy and levels of activity
– Points in process where the product is at greatest risk
Generally, your environmental monitoring plan should consist of two parts.
Firstly, prepare a general monitoring scheme that aims to demonstrate the effectiveness of maintenance, housekeeping, operator discipline and compliance with established standards.
For the general monitoring schemes, you will choose sampling locations that adequately provide data on such parameters.
The locations should provide good coverage of the whole cleanroom and associated areas, such as
– Changing rooms,
– Airlocks
– Transfer hatches
– Preparation areas, etc.
Secondly, prepare a batch/process-specific scheme that aims to account for individual processes and provide batch-specific information on the potential for product contamination.
You can use these data as evidence in the batch disposition process and to demonstrate compliance with established standards.
For batch/process-specific monitoring schemes, you will choose sampling locations to reveal potential problems with process and/or product integrity.
Your sampling points should reflect the process flow pattern, and environmental monitoring should follow the product flow through the manufacturing area.
In particular, points in the process where the products and components are exposed to the environment should be considered.
Your sampling should generally include:
– Air sampling (active and passive),
– Hard surface sampling (at or adjacent to critical areas),
– Operators gown and gloves
– Liquid sampling (e.g. bioburden).
Cleanroom classifications and particulate limits?
Cleanroom classifications:
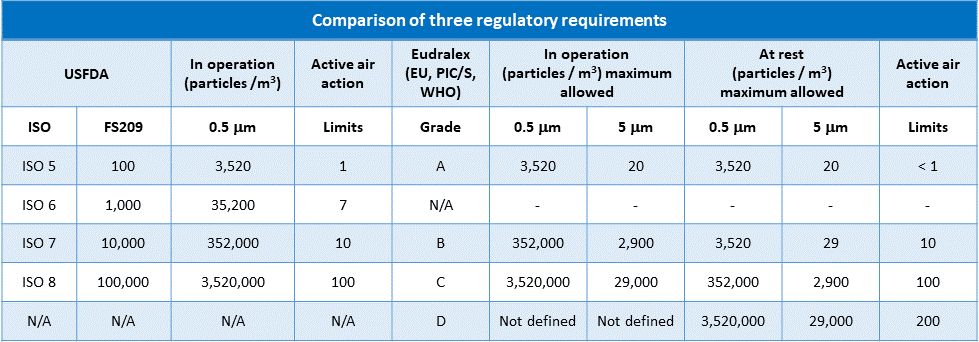
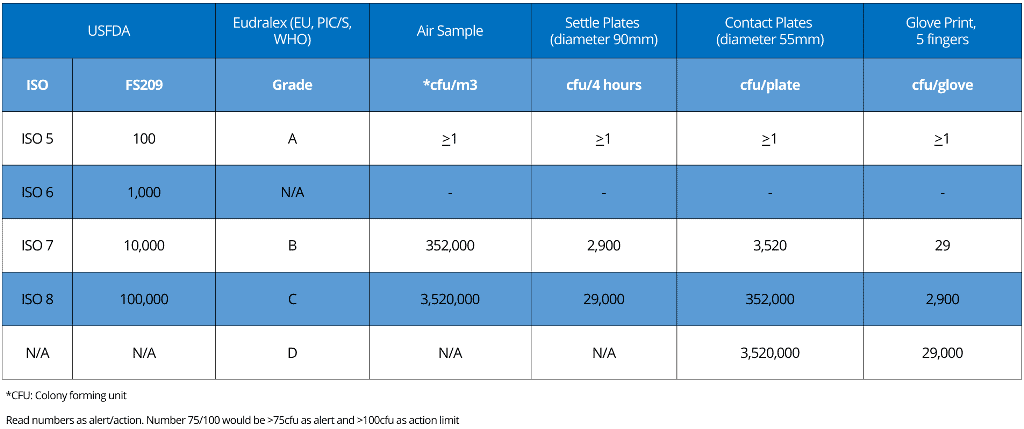
When you attempt to classify your cleanrooms and corresponding limits for non-viable airborne particulate counts, you may observe that some degree of misunderstanding and confusion exists between “2004 US Food and Drug Administration” (USFDA) environmental cleanliness requirements for sterile product manufacture and those of the “European Medicines Agency” (EMA) EudraLex Volume 4, Annex 1.
USFDA environmental cleanliness requirements are named “Federal Standard 209”.
The confusion has since been exacerbated by the USFDA’s adoption of ISO cleanliness classification system standards ISO 14644-1:2015.
Eudralex, Federal Standard 209, and ISO 14644-1 differ in detail but follow very similar numbers for the maximum permitted number of particles (limits) for each particle size.
Eudralex and Federal Standard 209 refer to alert as well as action levels.
With your needs in mind, we’ve dedicated our efforts to construct a comprehensive table below.
This table is designed to clearly demonstrate the differences and similarities between the three major regulatory requirements, providing you with a valuable resource for your work.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Please note:
i. FDA classifies cleanrooms from ISO 5 to ISO 8
ii. Eudralex classifies cleanrooms from grade A to D
iii. ISO 14644-1 specifies classes of air cleanliness in terms of the number of particles expressed as a concentration of air volume.
Particulate limits:
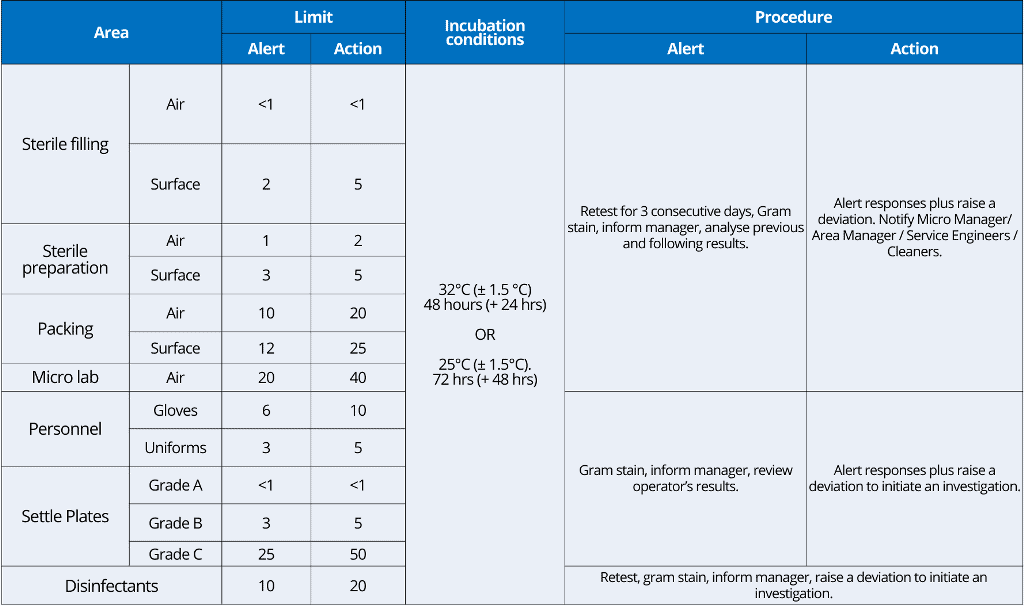
i. Alert Limit: Established microbial or airborne particle level, giving early warning of potential drift from normal operating conditions and triggering appropriate scrutiny and follow-up to address the potential problem.
Alert limits are always lower than action limits.
ii. Action Limit: Established microbial or airborne particle level that, when exceeded, should trigger an appropriate investigation and corrective action based on the investigation.
ISO 14698 also requires the establishment of a target value. This is value indicates the normal controlled performance of a location.
Sampling frequency examples:
Your plan for sampling frequency in environmental monitoring should depend on historical performance as well as changes to processes, plants, and procedures.
Normally, general sampling schemes are executed weekly, and specific sampling schemes are used during each work session.
You can use the following example of sampling frequency of environmental monitoring in aseptic processing.
– Settle plate monitoring of the filling rooms will be completed for each session of each fill operation, with a maximum of four hours of exposure.
– Each filling operator will perform glove prints and contact plate sampling of operator gowns in the Grade A (ISO 5) area before leaving the room.
– Any other staff in the filling room will perform glove prints and contact plates before each exit.
NB: Glove prints or contact plates for gowns are not required at exit when the room is opened, e.g., for cleaning, maintenance, and certification activities.
– Viable air sampling will be completed daily using suitable equipment (e.g., Merck MAS-100). Class A (ISO 5) areas will not be sampled during the filling machine’s operation.
– Non-viable particle counts will be completed continuously for each fill operation.
– RODAC plate sampling other than gown samples will be performed at the end of each fill day.
– At Rest, Environmental Monitoring will be completed one (1) day per six months and after major maintenance operations, e.g., preventative maintenance, certification activities, etc.
Your sampling plans should be approved, justified and contain clear schematics showing sample locations. Sampling procedures should contain information regarding when testing should be performed, by whom, under what activity and method to use.
Samplers should be appropriately trained (e.g. sampling may require excellent aseptic technique in order to avoid false positives).
Other key elements to consider in environmental monitoring plan
1. Temperature and relative humidity monitoring
You should control and monitor your facilities’ temperature and relative humidity as appropriate. Both of these factors should be connected to an alarm system in the processing area.
If the temperature and humidity are elevated, personnel may begin to shed increasing numbers of skin cells and sweat, causing more potential contamination.
An approved SOP or procedure should explain what actions to take if a temperature or humidity excursion occurs.
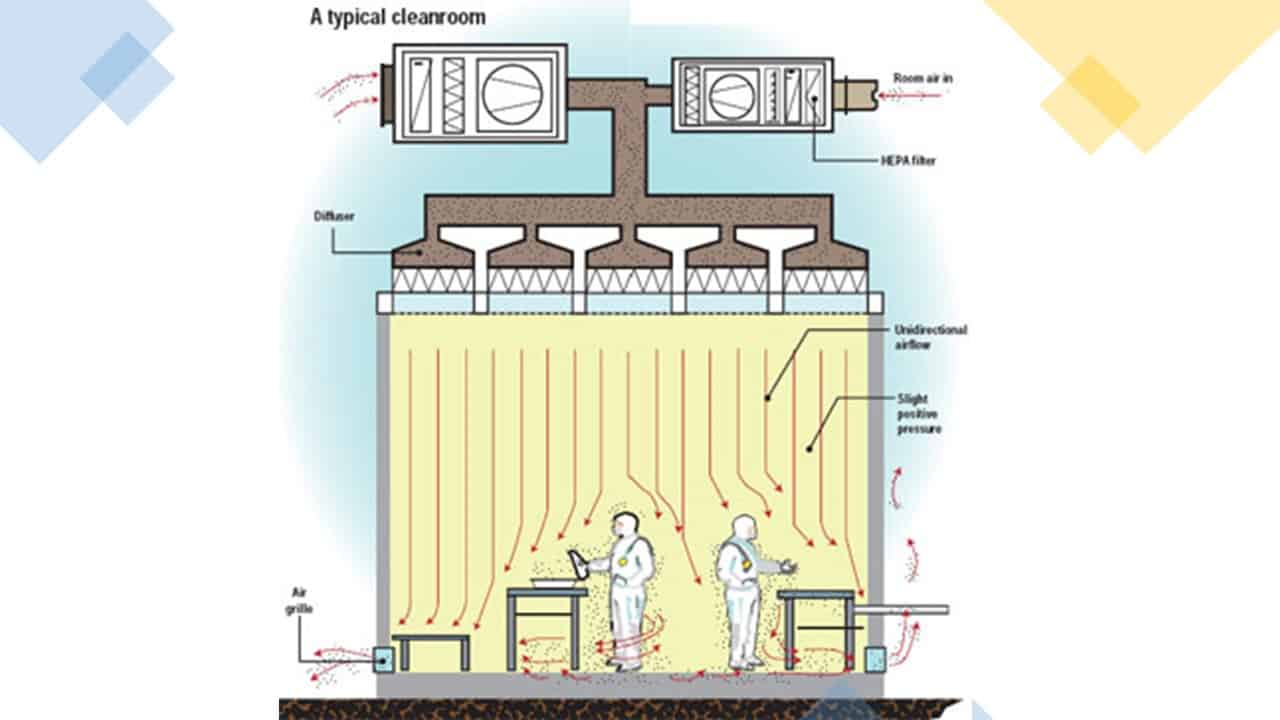
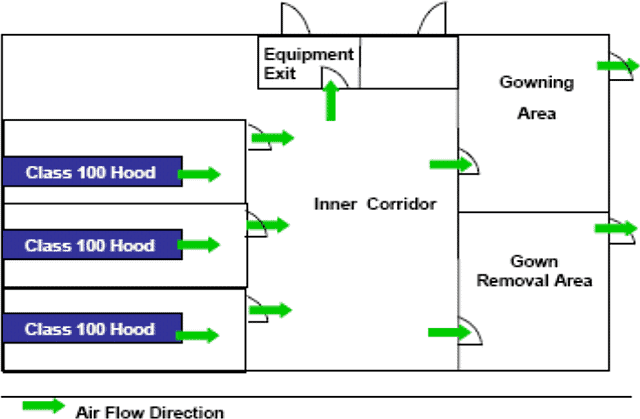
2. Pressure differentials
Appropriate pressure differentials from room to room and area to area are necessary to prevent contamination of the drug product.
You should maintain a positive pressure differential between the most critical and next critical areas.
Pressure differentials should be monitored and alarmed continuously.
A typical pressure and airflow for the area should look something like that shown below:
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
The flow of air should be out of the critical areas into the adjacent areas unless the opposite is required for safety or cross-contamination reasons.
The critical areas where the product is exposed should also be the cleanest areas, with the fewest numbers of particles and microbes.
3. Utilities:
Regular monitoring of utilities, such as the HVAC system, water system, and compressed gases, is of utmost importance.
These utilities can come in contact with the product or the air it is exposed to, making their monitoring crucial for maintaining product safety and quality.
– Monitor compressed gases by testing for viable and non-viable particulates.
– You should monitor the Water for Injection (WFI) system routinely. Tests that should be performed include microbial quality and endotoxin tests as well as USP chemical tests. The reason for testing this WFI is that it may be used as a solvent for the preparation of parenteral solutions and also for final product formulation.
– You should also test the potable water, purified water, the feed water for WFI, and component rinse water on a regular basis. The results of the tests should be analyzed for trends that might be developing.
What environmental monitoring testing are performed in pharmaceutical industry?
You need to divide the environmental monitoring testing into two major categories. Those are
1. Non-viable contaminants testing, and
2. Viable contaminants testing.
1. What is non-viable contaminant testing?
You should test the count of non-living particulates or particles that may contaminate a sterile product.
These would include dust particles, dirt, rust, tiny metal shavings, lint from a garment, human particulates, and other sub-visible particles that can still contaminate a sterile product if they are present in the environment.
Factors that are tested for this category of contaminant include:
1. Air in the processing and support area
2. Compressed gases
Be aware that the most critical processing areas, where sterile products or components are exposed to the air during processing, should be monitored for non-viable particulates.
Non-viable air sampling should routinely be conducted during aseptic processing.
You should place the sampling devices in grade A areas within one foot and directly upstream of the working zone where exposure to critical items or materials occurs.
Place the sampling device to sample the HEPA-filtered air just before it reaches the areas where critical items or materials are exposed.
Placement of the sampling devices in other areas of the processing suite is less prescriptive since these areas are usually subject to turbulent flow.
The frequency of the air testing should be every time the aseptic processing area is in use. Support areas may be tested less frequently.
Please look at the table for particulate limits for alert and action limits for non-viable monitoring.
2. What is viable contaminant testing?
You are required to perform viable or microbiological particulates/microorganisms in your processing environment.
Develop a microbiological isolate identification program. The extent and frequency of identification should be risk-based, with the greatest emphasis on any isolates from critical zones being identified.
Several methods are used to determine if microorganisms are present.
Some of the most common are:
Surfaces testing
1. Contact plate method
2. Swab method
Air Testing
1. Settling Plate method
2. Impaction/Impinger methods
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How to monitor surfaces for viable contaminants?
Ideally, you would need to monitor surfaces while the cleanrooms operate.
You should consider the type and level of activities that represent actual filling processes.
Some surfaces may be tested during or before operations. They should be monitored every time the aseptic processing area is used.
Critical surfaces within the filling area and personnel testing should be included as part of surface testing.
However, surfaces in critical zones should only be monitored after operations are completed due to the risk of introducing microbiological culture medium into the environment where critical activities are taking place.
Monitoring surfaces in surrounding areas during operations also poses the risk of leaving a residue of microbiological culture medium on the sampled surface.
You should only monitor these areas where necessary and with strict controls.
You should perform additional surface testing randomly, where personnel frequently contact the processing area to identify potential contamination sources.
Examples are curtains surrounding the Class 100/grade A area, door plates, phones, and tools.
1. Contact plate method:
The contact plate, or RODAC (Replicate Organism Direct Agar Contact) plate, consists of a solid general nutrient agar media approximately 25cm in diameter.
The sample is taken by gently rolling the raised surface of the agar plate onto a flat or slightly curved surface for a defined time interval.
After you conduct the test, wipe the area using a lint-free wipe soaked in disinfectant (isopropyl alcohol, IPA) to remove residual agar.
The plate is covered and incubated at an appropriate temperature.
The appearance of colonies on the surface of the plate detects the presence and number of microorganisms.
USE:
1. Personnel gloves and gowns
2. Filling room surfaces
3. Determining the effectiveness of cleaning and sanitization procedures
2. Swab testing method:
In the swab method, a sample is obtained from small or irregularly shaped objects or surfaces.
Samples are collected by swabbing the surface or object with a moistened sterile swab containing sterile diluent to ensure uniform coverage of the sampled area.
The swab is then transferred into a container with growth medium, which is then incubated at an appropriate temperature.
USE:
– Swabbing small items in the filling room
– Swabbing the interiors of small items
How to monitor air for viable contaminants?
You can sample microbes in the air in several ways. Depending on the requirements of the governing regulations and guidelines, passive sampling (e.g., settle plates) or dynamic sampling (e.g., impaction sampling) may be used to monitor the air.
Below are some methods used for monitoring air for viable contaminations:
1. Settle plate method:
An open petri dish of a defined size, agar media, and fill amount is placed within the filling area for a defined period.
The type and size of the settle plate generally determine this period. However, the norm is for exposure of 4 hours.
Particles form the air, fall onto the open plate, and settle on the agar surface.
At the end of the sampling interval, the plate is then incubated at pre-determined temperatures based on the common flora normally recovered at the local facility.
2. Impaction method:
You can collect samples using one or a combination of the three methods described below, which are called impaction methods.
All of these methods are considered dynamic because air is drawn into a sampling chamber to be collected.
The methods are generally used for critical processing areas
1. Slit-to-agar method:
This is considered an active sampling method. Through the use of a vacuum pump, usually a 1 Ft. area is pulled in through a slit with a calibrated width and impacted onto a slowly moving agar plate under the slit.
After the sample is taken, the agar plate is incubated, and colonies are counted.
2. Centrifugal method:
The sampler consists of a propeller that pulls a known volume of air into the unit and then propels the air outward to impact onto a nutrient agar strip.
After the sample is taken, the agar plate is incubated, and colonies are counted.
3. Sieve method:
The sampler is attached to a vacuum source that pulls room air, at a defined rate, through a top perforated with calibrated holes.
The air is then impacted directly onto an agar plate and exhausts through the vacuum line. After the sample is taken, the agar plate is incubated, and colonies are counted.
How to monitor operational personnel?
The last factor in the environmental monitoring system is personnel who populate the processing area.
Your personnel must know and follow their site’s gowning procedures in an aseptic area (ISO5 / USP 100 /Grade A).
You’ll need to prepare a formal, documented gowning training program. This program should include training in correctly putting on sterile garments and periodic refresher training.
This is necessary because each individual brings particulate and microbial contamination into the processing area.
You will need to be regularly tested in conjunction with activities performed in the aseptic processing area.
The EC Guide to Good Manufacturing Practice – Revision to Annex 1 states that surfaces and personnel should be monitored after critical operations.
You can use RODAC (contact) plates during personnel testing, usually on their gloves and in different places on their gowns.
You should be monitoring the personnel as soon as possible after completing critical tasks.
You are expected to establish limits based on recommendations from worldwide regulatory documents as well as data collected from monitoring.
Conclusion
All of the factors mentioned above need to be aligned and integrated to produce a clean environment for processing sterile drug products.
Each factor plays a vital role, and if any one of them is not within limits or is not working properly, it can have a significant impact on the entire processing environment, potentially jeopardizing the sterility, integrity, potency, and quality of the final drug product.
The goal of the environmental monitoring program is to control contamination through the rigorous monitoring of all of these factors and prevent loss of control through early detection.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.

I’m a retired microbiologist,and noticed a relatively new product type on the market called probiotic cleaners. “Good bacteria “are sprayed to clean up bad bacteria (this is insane). Situational awareness should be raised regarding this product type lest it be inadvertently used by one of us. What do you think of probiotic cleaners?
Wow! This is really interesting news and worth to explore further.
Pl share QRM for if EM by air sampling is not performed