Basic cleaning and sanitation practices in Pharmaceuticals
- Published on: Feb 29, 2024
Cleaning and sanitation of pharmaceutical equipment and facilities account for almost one-third of all production activities, if not more.
Uncompromised safety and efficacy of pharmaceutical products are the best outcomes for the patients. Cleaning and sanitation steps hold the key to reaching that outcome.
Anyone who works in pharmaceutical production is familiar with the fear of unexpected contamination of their products.
To prevent such disasters from happening, pharma employees take countless measures, including best-of-class cleaning and sanitation steps.
Yet, each year, destroying production batches for actual or suspected contaminated products is expected.
According to the National Library of Medicine’s 30-month analysis of FDA drug recalls, contamination is among the top five reasons for drug recalls.
Effective cleaning and sanitation practices involve using appropriate disinfectants, cleaning agents, and sterilization techniques to remove any traces of residue, microbes, or foreign particles from equipment, surfaces, and production areas.
This helps prevent cross-contamination and minimizes the risk of compromised product quality and safety.
This article will explore the key considerations, best practices, and regulatory requirements for effective cleaning and sanitation that pharmaceutical companies must adhere to for optimal cleanliness and safety.
Regulatory requirements for cleaning and sanitation in pharmaceuticals
For more than a few decades, it has been an essential GMP requirement that all cleaning and sanitation steps in a pharmaceutical plant be documented accurately and validated to prove they work.
Once the cleaning steps are validated, employees must only follow the approved procedure.
According to UDFDA, Equipment – Subpart D Sec. 211.67 Equipment cleaning and maintenance.
(a) Equipment and utensils shall be cleaned, maintained, and sanitized at appropriate intervals to prevent malfunctions or contamination that would alter the drug product’s safety identity, strength, quality, or purity beyond the official or other established requirements.
(b) Written procedures shall be established and followed for cleaning and maintaining equipment, including utensils used in manufacturing, processing, packing, or holding a drug product. These procedures shall include, but are not necessarily limited to, the following:
(1) Assignment of responsibility for cleaning and maintaining equipment;
(2) Maintenance and cleaning schedules, including, where appropriate, sanitizing schedules;
(3) A description in sufficient detail of the methods, equipment, and materials used in cleaning and maintenance operations and the methods of disassembling and reassembling equipment as necessary to ensure proper cleaning and maintenance;
(4) Removal or obliteration of previous batch identification;
(5) Protection of clean equipment from contamination before use;
(6) Inspection of equipment for cleanliness immediately before use.
(c) Maintenance, cleaning, sanitizing, and inspection records shall be kept as specified in 211.180 and 211.182.
Some kinds of contamination are often difficult to detect by physical inspection.
The claim: “It looks clean. Therefore, it must be clean,” is not necessarily true.
Laboratory testing for potential contaminants is generally not practical, economical, or reliable.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
What is cleaning?
Cleaning is the removal of visible and microscopic contamination by dirt, extraneous matter, or product residues by mechanical or physical means.
After cleaning a piece of equipment the first thing you should do is to perform visual inspection.
When you are confident that cleaning has removed all visual contaminants, take representative rinse or swab samples and send them for laboratory testing to verify that the cleaning process continues to be effective.
Effective cleaning usually requires using cleaning agents such as detergents and solvents, which are used under specified pH, temperature, time, and solvent concentration conditions.
A cleaning concern is whether or not the cleaning agent itself will have a negative effect on the life of the equipment or the quality of the product.
What is sanitation?
Sanitation is the reduction of microbiological contamination. It is usually achieved by the use of sanitizing agents.
However, it can also be achieved by heating, steaming, and even vigorous mechanical action, such as scrubbing.
Note: Cleaning may result in a partial reduction of the microbial load. To reduce any microbial load under an acceptable limit, you have to sanitize your equipment.
If sanitizing chemicals are used, it may be necessary to remove the sanitizers’ residues by further cleaning.
So, it is very important to use the specified amount of sanitizing agent.
Several factors determine how effective sanitizing will be. These include:
– The number and types of microbes
– The type of material containing the microbes
– The concentration, temperature, and pH of the sanitizing agent
– The length of time you sanitize.
It is impossible to sanitize a dirty surface. The surfaces must first be thoroughly cleaned.
How cleaning and sanitation prevents contaminations
By now, you have realized that a combination of cleaning and sanitation is needed to reduce all potential contamination effectively.
Cleaning itself does half the job. It removes visible contaminants. Sanitation will remove the invisible microorganisms.
Combining these two in a balanced way is the solution to eliminate most contaminations.
Take the following examples from pharmaceutical processing areas that demonstrate the power of cleaning and sanitation to reduce contamination.
1. After a production run, you clean and sanitize the equipment. After cleaning, you collect rinse or swab samples from critical equipment surfaces and send those for laboratory analysis to verify that residual levels are within acceptable limits. This process ensures that remnants of previous formulations do not contaminate subsequent batches.
2. In tablet manufacturing, the compression tools are dismantled for detailed cleaning of punches and dies. This prevents cross-contamination between different formulations and tablet batches produced using the same compression machine.
3. Equipment surfaces are often treated with anti-static agents such as quaternary ammonium salts to minimize the buildup of electrostatic charges, reducing the risk of particle adhesion.
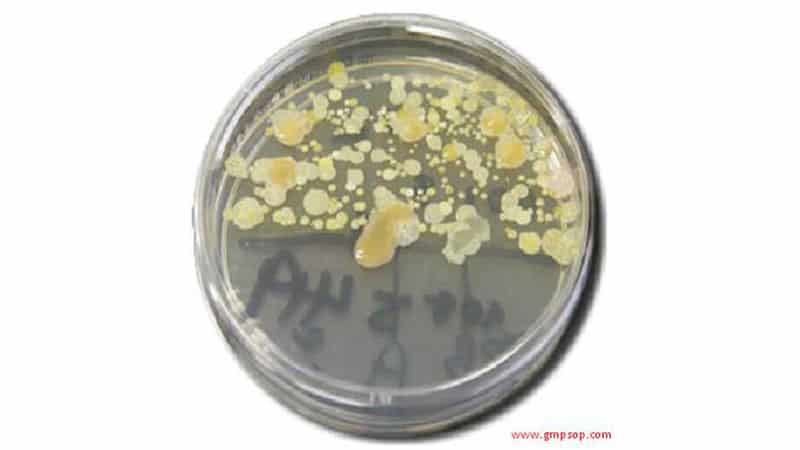
4. During environmental monitoring, ager plate samples are taken from the air, contact surface, operator gloves, and gowning. The plates are then incubated to detect and count microbial colonies. The alert and action limits indicate whether cleaning and sanitation practices effectively reduce contamination.
5. During the production of sterile products, Clean-in-place (CIP) systems are commonly used to clean stainless steel tanks and piping between batches. This kills the bioburden loads and minimizes the risk of contamination from harmful microbes.
6. Sporicidal disinfectants are used in the cleanroom environment to eliminate bacterial spores on surfaces.
7. Sometimes, pharmaceutical manufacturers employ surface profilometry to assess the roughness of stainless-steel equipment. Smoother surfaces are generally easier to clean and less likely to harbor contaminants.
8. At the entrance of production areas, employees may step onto sanitizing mats that dispense a disinfectant solution, reducing the likelihood of foot-borne contamination.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Different types of contaminants in pharmaceuticals
As a pharmaceutical product manufacturer, you must constantly fight against contaminants in chemical, physical, or microbiological forms.
Some familiar sources of contamination in pharmaceutical sites are:
1. Chemical contamination
Chemical contamination occurs when the product becomes contaminated with other foreign materials, products, or residues.
This can occur when different products are processed in the same equipment or when materials such as cleaning agents and lubricants come into contact with products or equipment surfaces.
The way to avoid chemical or biological spills or contamination is by following written cleaning procedures strictly.
Often, you have to validate the steps and conditions in the procedures to ensure they are effective every time under different conditions.
Changing the steps or conditions may make the cleaning process ineffective.
2. Environmental contamination
Different manufacturing areas within the facility carry different levels of contamination risk to the product.
There is a higher risk of contamination in areas where the product is exposed to the environment, for example, in the dispensary, formulation areas, and bottle-filling rooms.
To reduce environmental contamination, you must take necessary measures to control the air, typically using filters.
Frequent facility cleaning and environmental monitoring are strongly recommended.
In other areas, such as secondary packaging or material storage areas, the product isn’t directly exposed to the environment, so there is a lower risk of contamination.
In such areas, a lower level of environmental monitoring will be sufficient. You can reduce the frequency of cleaning for these facilities.
3. Biological Contamination
Biological contamination refers to contamination by bacteria, yeasts, molds, viruses, or any other microorganisms that may be present in the product.
This also can include residues from dead micro-organisms or endotoxins, which can contaminate injectable products.
Biological contamination’s origins vary but include the environment, personnel, raw materials, contaminated water, and poorly cleaned or wet equipment.
While cleaning reduces biological contamination, sanitizing agents play an important role in killing microorganisms.
When you are in business to prepare sterile products, production must be conducted in cleanrooms protected by High-Efficiency Particulate Air (HEPA) air filters.
Cleanroom requirements are very strict in these areas and are usually associated with positive room pressures (overpressure) to protect the areas from external airborne contamination.
Usually, 99.97% of all airborne particles above 0.5 microns are excluded by the HEPA filters.
The prevention of cross-contamination is particularly important when you produce products such as steroids, hormones, antibiotics, cytotoxic products, and some potentially pathological biological materials.
These materials, even in minute amounts, may have a significant effect on the body, and it is important to isolate them from the environment during manufacture and remove their residues from equipment during cleaning.
If you manufacture any of these products, ensure that you are fully aware of the rules for handling and cleaning. Wear the protective gear provided.
Visual inspection of products is easy and inexpensive, but it is often labor-intensive and unreliable.
Although obvious contaminants can be seen, there may be microscopic contaminants in the product, such as microbial, particulate, and product chemical residues, which are all difficult and expensive to detect once inside the product.
Good cleaning and sanitation practices aim to prevent contamination from arising first rather than try to detect it once it is present.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Types of cleaning and sanitizing agents
Your selection of cleaning and sanitizing agents will depend on factors and conditions, including the type of equipment, the nature of residues, hard-to-clean areas, pipework, temperature, pressure, etc.
You must establish the effectiveness of cleaning and sanitizing agents through validation trials.
Following is a list of commonly used cleaning and sanitizing agents in pharmaceutical equipment cleaning:
Alkaline Cleaners: These are effective against fatty and oily residues. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
Acidic Cleaners: Useful for removing mineral deposits and scale. For example, Citric acid, phosphoric acid, or nitric acid.
Detergents: General-purpose cleaning agents for removing a wide range of residues. For example, non-ionic or anionic detergents.
Solvents: Solvents are used for dissolving and removing organic residues. (e.g., Isopropyl alcohol, ethanol, acetone, or ethyl acetate).
Chelating Agents: Bind and remove metal ions, preventing their interaction with other residues. An example is ethylenediaminetetraacetic acid (EDTA).
Enzymatic Cleaners: these are used to break down biological residues such as proteins, fats, and carbohydrates (e.g., Protease, amylase, or lipase enzymes).
Surfactants: Reduce surface tension, aiding in removing dirt and residues. (e.g., Tween, Triton X-100, or sodium lauryl sulfate.
Ammonium Compounds: Quaternary ammonium compounds, such as Benzalkonium chloride, act as disinfectants and surfactants.
Phosphoric Acid-Based Cleaners: Effective against mineral deposits, such as Phosphoric acid-based solutions.
Purified water or water for injection (WFI): Ensures complete removal of cleaning agents after cleaning.
Aldehyde-Based Cleaners: Glutaraldehyde or formaldehyde-based solutions.
Different sanitizing agents will be effective only in certain circumstances. We have categorized four types of sanitizing agents commonly used in pharmaceutical manufacturing.
1. Sanitizers
Proper use of sanitizers can reduce bacteria by >99.9% if used on pre-cleaned surfaces. Pre-cleaning is always required to reduce bioload and dirt.
2. Disinfectant
Proper use of disinfectants can result in 100% killing of vegetative bacteria, target viruses, and target fungi. Pre-cleaning may or may not be required, but it is considered a pre-requisite.
3. Sterilant
Proper use of sterilants can result in 100% killing of all microorganisms, including bacterial spores. Pre-cleaning is always required to reduce bioload and dirt.
4. Sporicide
Proper use of sporicides can result in a 100% killing of all bacterial spores.
Following are some common sanitizers of choice used in GMP facilities.
– 70% v/v alcohol (for surfaces)
– Quaternary ammonium compounds (QUATs)
– Phenolics
– Sporicides (for killing spores)
– Chlorines (such as hypochlorite)
– Hydrogen peroxide/peracetic acids
– Sodium hydroxide (cleaning & partial sanitizing)
– Vaporized Hydrogen Peroxide (VHP)
You should be familiar with your choice of sanitizing agents and only use approved agents in the nominated strengths.
Sterilization Vs sanitation
Sterilization means the total elimination of any living organisms, usually by heat or chemical means, of any living organisms.
Sanitation only reduces the microbial load (the bioburden) by chemical means. The result of sanitation does not guarantee the complete absence of microorganisms.
Most chemical and physical agents will clean, sanitize, and disinfect production equipment but not sterilize.
Sterilization is your only choice when you intend to produce sterile products free of microorganisms. Sterilization reduces the probability of the presence of viable microorganisms to an acceptable extent.
Sterilization can be achieved by moist or dry heat or treatment with a gaseous sterilant such as ethylene oxide.
Sterilization also includes other methods, such as irradiation with ionizing radiation or, where such processes are inapplicable to solutions, by filtration.
Some standard sterilization techniques that are typically used in pharmaceuticals are:
1. Steam Sterilization
This technique uses steam at 121°C to sterilize fluids in ampoules, vials, etc. This is the first choice of sterilization method for processing equipment, reactors, preparation tanks, solution delivery piping, etc.
Sterilization by applying saturated steam under pressure is generally the preferred method. The principles apply to sterilization-in-place (SIP) processes.
2. Sterilization by filtration
This technique is used for those products that cannot be sterilized due to the heat sensitivity of the product or where heat labile packaging is chosen since it provides a distinct patient benefit.
However, this is usually not the preferred sterilization method.
3. Dry heat sterilization and depyrogenation
This technique is commonly used to sterilize/depyrogenate containers (ampoules, vials, etc.), pharmaceutical raw materials, and processing equipment.
I observe that dry heat has little application for sterilizing pharmaceutical drug products.
4. Radiation sterilization
This is used to sterilize packaging equipment, consumables, garments, etc., that are difficult to sterilize using steam or other methods.
Radiation sterilization is mainly used for medical devices.
GMP rules for cleaning and sanitation
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
The GMP rules are very explicit regarding the requirement to clean and sanitize equipment and the manufacturing facility.
Since most residues cannot be seen nor reliably tested for, your company’s standard procedures and cleaning records are relied upon as evidence of cleaning.
1. Cleaning and sanitation procedures
GMP requires the cleaning procedures to be fully documented in written procedures.
These procedures are initially validated (shown to be effective) specifically for an item of equipment or an area.
Once validated, the procedures are then published and used.
2. Cleaning validation
All cleaning procedures should be validated (shown to be effective) under specific conditions of use.
These conditions of use are specified in the written procedure.
If employees alter the conditions of use, the cleaning methods may be “invalidated” and ineffective.
For example, it would seem logical that adding more sanitizer than the procedure requires would make cleaning faster or better. This is not always the case.
Adding additional sanitizer may alter the pH of the cleaning agent and make it less effective.
The relevant GMP rules are to only clean under validated conditions and strictly follow procedures.
3. Cleaning and sanitation conditions
The written procedures describe the required conditions under which cleaning is optimal.
These conditions may include a range of the following:
– Strength of the cleaning or sanitizing agent (e.g., 2.0% v/v)
– The type of water or solvent to be used
– The pH of the cleaning agent
– The temperature of the water to be used
– How much to dismantle equipment before cleaning commences
– The contact or residence time for the sanitizing agent on the surface
– What agents to use to clean off the sanitizing agent
– What standard of water to use in the final rinse (GMP, for example, requires purified water for injection as the final rinse)
4. Cleaning and sanitation records
One essential GMP rule is the keeping of detailed cleaning records. Cleaning records prove that cleaning took place and evidence of the cleaning outcomes.
For example, the surfaces are visually clean or the results of rinse water tests.
The records must also identify who did the cleaning and when and must be signed.
For automatic cleaning procedures such as clean in place (CIP), the cycle conditions are usually automatically monitored and the conditions recorded.
Cycle conditions may include the temperature, flow rate, time, concentration of solvent, and solvent agitation time. Often, CIP systems have alarms when something goes wrong.
The completed and signed records should be attached to the records since they provide the only objective evidence that cleaning has occurred.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
What must be cleaned?
– The facility must be regularly cleaned. Particular emphasis is placed on areas or rooms where product is processed.
– Particular emphasis is placed on effectively cleaning all equipment in contact with the product, and it must be cleaned according to validated and detailed procedures.
– All equipment in processing areas not in contact with the product needs regular cleaning to prevent dust and dirt buildup.
– The inside of the factory should be regularly cleaned, but particular attention should be paid to the processing and storage rooms. The floors, surfaces, benches, walls, and ceilings should undergo cleaning at defined intervals.
– Cleaning and sanitation also apply to any equipment in contact with the product during manufacture, such as production and packaging equipment, transfer lines and tanks, and storage containers and drums.
Protecting yourself during cleaning and sanitation
Protecting yourself is one of the most overlooked areas during cleaning and sanitation.
Chemicals may cause adverse reactions in people if they are continuously exposed to them.
Regular and thorough cleaning, wearing protective garments, and abiding by correct handling procedures eliminate this problem.
– Wearing hair nets.
– Wearing protective gloves and face masks.
– Wearing disposable gowns.
– Checking the procedure to verify if the chemical residues need special handling.
Conclusion
If you’re worried about unexpected contamination at your GMP facility, it’s important to know that implementing a thorough cleaning and sanitation program can help alleviate your concerns.
The article discusses the significance of cleaning and sanitation practices in reducing contamination in products, regulatory requirements for cleaning, different types of contamination, and the cleaning and sanitizing agents you should use.
It is crucial to identify and prevent the three main types of contamination – chemical, environmental, and biological – which showcases the meticulous approach required in pharmaceutical cleaning.
While it is important to follow validated cleaning procedures, it is equally important to balance this with flexibility.
Deviation from documented conditions can compromise the effectiveness of the cleaning methods, putting drugs at risk of contamination during production.
Areas such as the dispensary, formulation areas, and bottle-filling rooms are more exposed to the environment and therefore require heightened attention and more frequent cleaning, with additional measures like air control and environmental monitoring.
Ultimately, the success of pharmaceutical manufacturing depends on meticulous cleaning and sanitation practices.
By understanding and implementing the regulatory guidelines, the industry can ensure the desired level of cleanliness and sanitation, ultimately contributing to producing safe and high-quality medicines that meet regulatory standards.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
Chemical or Biological Spill Response Procedure
Surface sampling swabbing method for microbiology tests
Quality Agreements with Third Party Manufacturer