Basic Overview of Contamination Control in GMP Facility
- Kazi
- Last modified: March 15, 2025
Contamination control is taken extremely seriously by the GMP facilities as it can adversely affect the quality and safety of food and drugs they produce.
To control contamination, GMP facilities have to implement strict hygiene and sanitation procedures, robust cleaning programs, use dedicated equipment & facilities, carefully design production lines and material storages, perform regular quality control tests and QA release activities throughout the supply chain.
Table of Contents
How to define contamination and cross-contamination?
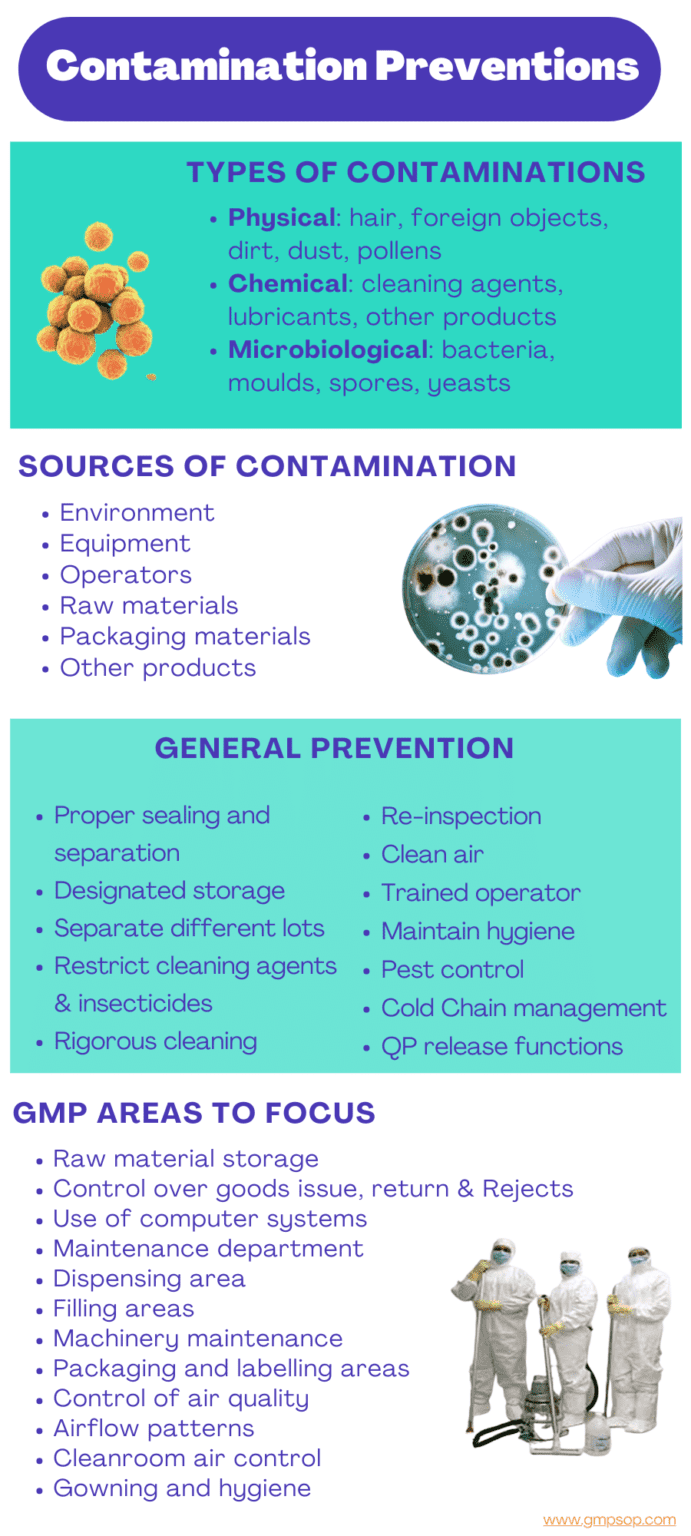
Contamination is the presence of any foreign substance in our products. It may be:
– Physical: hair, foreign objects, dirt, dust, pollens
– Chemical: cleaning agents, lubricants, other products
– Microbiological: bacteria, moulds, spores, yeasts
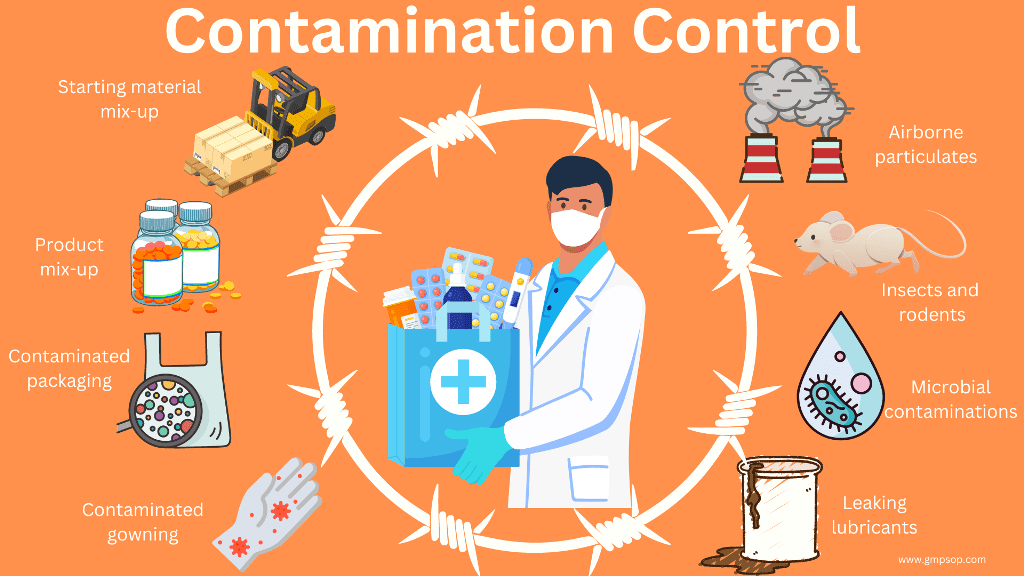
Cross-contamination of product is caused by either:
– Contamination of a batch with a previous batch of the same product
– Contamination with a different product through carryover or proximity of production lines
– Contamination by a foreign starting material usually of the dispensary or in the store.
As an example, if you have worked in a tablet filling line, you may have occasionally found fragments of tablets which are of different sizes, shapes, colors and active ingredients under the vibrating tablet feeder tray.
If the tablet feeder area is not cleaned regularly, sometime after each batch of filling, one of those rogue products can cause cross contamination to next batch.
Line cleaning and line clearance process can effectively eliminate the risk of contamination. Production line Operators have to wear special gowns, head cover, gloves and masks to prevent potential cross contamination.
Separation of production lines, warehouse storage areas, material or personnel flows are strictly controlled for the prevention of cross contaminations.
How to prevent cross-contamination in GMP facility?
You must take numerous preventive measures to reduce or eliminate cross-contamination risks in GMP facility. A summary of those can be listed as follows:
a. Maintain proper sealing, separation and storage of raw materials.
b. Take adequate care in management of the dispensary so as to exclude the opening of different lots of containers in close proximity.
c. Employ thorough and rigorous cleaning of all equipment, utensils, transfer lines, extraction systems and vessels after use.
d. Re-inspect all equipment before use and line clearances at all stages of manufacture.
e. Ensuring all air conditioning systems are serviced and property maintained.
f. Have a well-designed and operated facilities.
g. Implement good housekeeping practices.
h. Develop written procedures for:
– Handling and storing material and products
– Cleaning of equipment and facilities
– Preventive maintenance programs
– Conducting room and line clearances
– How to train your personnel on GMP and specific duties
– Documenting and investigating all deviations or abnormalities
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How to take contamination control measures in GMP?
Regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) has published detailed guidance and training on contamination control due to its serious implication on product quality and potential for human harms.
We will touch on some of the common measures GMP facilities can take as part of controlling contamination.
a. Contamination control tests in laboratory
There are various types of analytical tests that are used in GMP facility, including:
– Identity testing – to confirm the identity of a drug product and ensure that it is not counterfeit.
– Purity testing – to determine the presence of impurities, contaminants or foreign substances in the product.
– Potency testing – to determine the strength or concentration of the active pharmaceutical ingredient
There are several chromatographic and spectrophotometric tests that are commonly used to detect contamination in GMP facility, including:
i. High-Performance Liquid Chromatography (HPLC) – used to identify and quantify contaminants in a sample.
ii. Gas Chromatography (GC) – used to analyze volatile components in a sample, including potential contaminants.
iii. Mass Spectrometry (MS) – used to identify and quantify contaminants in a sample with high precision and accuracy.
iv. Nuclear Magnetic Resonance Spectroscopy (NMR) – used to identify and quantify impurities in a sample based on their unique molecular structure.
v. Infrared Spectroscopy (IR) – used to identify and quantify the presence of impurities in a sample based on their characteristic infrared absorption spectra.
vi. UV-Visible Spectroscopy – used to quantify the concentration of contaminants in a sample based on their absorption of ultraviolet or visible light.
In addition to analytical tests, several physical tests are also conducted to detect contamination in GMP facility. Some of the commonly used physical tests include:
i. Visual inspection – examining the appearance of the product to check for any visible signs of contamination, such as discoloration, sediment, or foreign particles.
ii. Particle size analysis – determining the size and distribution of particles in a sample to check for any unwanted impurities or contaminants.
iii. Texture analysis – examining the physical properties of the product, such as hardness, cohesiveness, and particle size, to check for any inconsistencies that may indicate contamination.
iv. Microbial tests – culturing a sample to check for the presence of microorganisms, such as bacteria or fungi, that may indicate contamination.
v. Physical Stability tests – monitoring the physical characteristics of the product over time, such as size, shape, and color, to check for any changes that may indicate contamination or degradation.
vi. Related substance test: related substance tests are carried out using chromatographic analysis to quantify impurities and degradation substances in pharmaceutical products.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
b. Contamination control in cleanrooms
The control of cross-contamination is accomplished by the use of positive air barriers (to exclude other products), dust extractors and containment hoods (to trap and remove dust) during processing.
Cleanrooms are specifically designed so the air flow patterns, and room pressures protect the product. It is very important to maintain this protection by:
– Keeping doors closed (to prevent loss of pressure)
– Keeping air returns clear (to maintain sweeping of air)
– Cleaning extractors and filters (for efficient operation)
– Checking room monitors (to verify set pressures)
– Regularly maintaining/servicing the air handling units
i. Main sources of contamination in cleanrooms
– Environment in which the product is formulated and filled
– Equipment used in formulation and filling
– Operator induced
– Raw materials and packaging materials
– Other products may cause cross-contamination
If you suspect that contamination has occurred, make a note on the batch records and tell your supervisor. This way, there is a greater chance of detecting the contaminant.
ii. Cleanroom air control
Air must be controlled in rooms where product is exposed to the environment.
Some important controls for cleanrooms are:
– Filter the incoming air
– Control the temperature
– Control the humidity
– Have fast flowing air
– Have a laminar flow
– Keep the room at a higher pressure
– Than the surrounding areas
– Keep doors shut!
iii. Airflow patterns and contamination control
There are basically two types of clean rooms:
1. Those designed to exclude particles, dirt, bacterial and other products – these are called positive pressure rooms where the room pressure is higher than the outer rooms.
2. Those designed to contain dust generated during product processing within the room – these are called negative pressure rooms where the room pressure is less than surrounding rooms. These rooms often have a dust extraction system to trap product dust.
The effective operation of either room type is dependent on keeping doors closed and ensuring inlet filters, air return vents and dust extraction equipment is clean and operating properly.
Positive pressure rooms
Positive pressure rooms are at a higher pressure than surrounding areas – if the door is kept closed. The rooms are designed to clean the air by exchanging it about every 3 – 5 minutes through air filters in the inlet vents. The air is swept across the room to outlets (returns) near the floor and door. Provided the doors are kept shut the room should be very clean.
These rooms are used when the exclusion of bacteria and particles from the room and product is important.
Products such as creams, oral liquids, ointments and sterile products require these cleanrooms during processing, where bacterial exclusion is important.
Negative pressure rooms
Negative pressure rooms are used when it is more important to trap or contain any product dust in the room or dust extractor. This is done to prevent contamination of other processing rooms and possible cross-contamination of other products and equipment.
Rooms of this type usually contain a secondary extractor to collect the product dust and sometimes filters on the return air vents and ducts. It is important to keep the cleanroom doors shut. Maintain the equipment regularly for effective extraction systems.
Dry oral products, such as tablets and capsules, where bacteria or particles are less of a problem, are usually processed in these rooms.
Work areas should be as close to the air inlet as practical since this is the location where the air will be the cleanest.
Air should flow away from the workstation and out of the room in a smooth manner.
Doors should be kept shut to maintain the air pressure in the room.
Do not obstruct the air outlet this will upset the flow of air.
It important to remember, always keep the air supply and returns area clear of obstructions. This way, you will minimize risk of environmental contamination.
c. Contamination control of raw materials store
Poor housekeeping in the store can lead to product mix-ups and cross-contamination. Take following measures to control the contamination:
– Doors should be kept shut to prevent dust and pests from entering.
– Materials should be stored off the floor in a way to prevent damage or contamination.
– Spills should be cleaned up immediately.
– All materials and products should be clearly labeled to prevent mix-ups.
– There should be separate storage areas for quarantined goods and a locked area for rejected materials.
– The open container is prone to contamination from humidity or other microbial agents in the environment.
– Fred is not wearing protective clothing.
– Poor housekeeping practices and lack of restricted access can lead to product mix-ups or cross-contamination.
d. Contamination control over incoming goods
Incoming goods need to be controlled. Each lot of material must be assigned a unique identification number to ensure traceability and checked for possible transit damage and contamination.
Once these materials are accepted into the facility, they must be stored in such a manner that contamination is minimized.
Before use, these materials must be checked for correct identity, cleanliness, integrity of the storage container or protective wrapping and suitability for use in the manufacturing process.
Only by these checks can operators satisfy themselves that the material has not been contaminated during storage end is in fact the right material to be using.
e. Controls with the use of computer systems
Often computer systems are used to control inspection and test status. In this case, materials and products may not have physical status labels or even be stored in separate areas. Such systems must be validated and thoroughly documented. All operators must be trained in the use of these systems,
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
f. Contamination control in maintenance department
Poor housekeeping practices by maintenance personnel can cause problems in manufacturing.
– Operators should observe requirements for wearing clean working gowns.
– Doors should be kept shut to prevent dust and pests from entering.
– Poor cleaning and hygiene practices can lead to contamination of product.
– Never use product bottles for lubricants or other supplies. Always label bottles clearly to prevent mix-ups.
– Spills should be cleaned up immediately.
– Pest control programs should be in place for the entire facility.
– This poor repair job has created a high risk for microbial contamination.
g. Contamination control in dispensary
Dispensing areas have been designed to minimize the risk of contamination and mix-up. It’s important that only raw materials are dispensed by authorized operators following standard procedures.
– Operators should observe requirements for wearing clean working gowns.
– Doors should be kept shut to prevent dust and pests from entering.
– These two drums should never be opened at the same time.
– There should be special precautions such as exhaust fans to prevent the generation of dust.
– Exhaust fans are only effective if they are well maintained and switched on. Cleaning procedures need to specify the methods for cleaning exhaust ducts, grilles, flues and fan blades.
– Dirty equipment can be a source of contamination.
h. Contamination control in product filling
Poor contamination control in the filling area can have disastrous consequences. To control contamination in this area, take the following measures:
– Doors should be kept shut to prevent dust and pests from entering.
– Operators should observe requirements for wearing clean working gowns.
– Air handling units should be installed, operated and maintained to ensure a clean supply of air.
– Never use product bottles for lubricants. Always label bottles clearly.
– Always wear the specified work garments to prevent cross-contamination.
– Poorly maintained equipment and incorrect machine set ups may damage product containers.
Never return dropped product to the line.
i. Control of contamination by machinery
The machinery used in manufacturing a product is an often-overlooked source of possible contamination.
Unlike some visible contaminations, e.g., bacterial contaminants, not all oil contaminants can be easily seen. So strict controls are needed to prevent oil contamination from equipment. You must know and follow your company’s procedures while cleaning equipment.
Possible contaminants are lubricating fluids, wearing parts that generate metal particulates, previous product remaining as a result of incorrect cleaning and malfunctioning equipment that smash containers or have inefficient extraction devices fitted.
The operator using these machines can ensure that contamination of product does not occur by ensuring that the machines are thoroughly cleaned after use and examined for cleanliness before use.
During operation, the operator should be constantly aware that a malfunctioning machine may contaminate product and be alert for teaks, drips from lubricating glands or wearing of machine parts.
Machines should be kept in good running order by having periodic preventative maintenance checks performed according to a schedule that relates to the machine age, use, and requirement for servicing.
A maintenance program that is directed at breakdown maintenance will not minimize the possibility of contamination of product.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
j. Contamination control through air quality
Contamination can occur if contaminated air comes in direct contact with the product at the dispensing of starring materials, the formulation of the product or the filling of me product into the final product containers.
In each case, the contamination may be airborne particulates, other products or a starting material of a previous product.
The sources of airborne contamination can be the air supplied to the facility, operators working in the facility and generating the contaminant, materials bought into the facility or machinery used within the facility.
Control of airborne contamination is achieved by controlling the quality of air within the facility. This relates back to the design of the facility, the air handing system and the rooms within the facility.
The type of air filtration employed within the facility and the air flow are important factors in controlling contamination.
Just as important is the temperature and humidity within the facility. A facility that is too hot or too humid rapidly causes operators to perspire and perspiring operators are a source of particulate and microbial contamination. For these reasons, processing rooms and storage areas should have controlled access.
The quality of air required in processing areas will be different for different products and for different manufacturing stages of product.
k. Contamination control in Packaging operation
Special emphasis needs to be given to the control of labels and pre-printed packaging materials to prevent mix-ups and product recalls.
– Operators should observe requirements for wearing clean working gowns.
– Doors should be kept shut to prevent dust and pests from entering.
– Labels should be kept in restricted areas. They should be kept in sealed containers and returned to the store before the next operation.
– Never take personal medication into the manufacturing areas.
– Operations should be segregated, and signs should clearly identify the product being packaged to prevent mix-ups.
– Different products must be segregated. Labels should be securely fixed to the container – not the lid.
– Prevent cross-contamination by conducting a line clearance before commencing work.
l. Contamination by people
GMP processing Operators are a major source of contamination.
Operators need to be disciplined in their work habits and understand and follow all procedures relating to the manufacture of product.
Operators involved in processes where starting materials or products are exposed should not be suffering from a contagious disease or have open lesions on the exposed surfaces of the body. If this is the case, report to your supervisor.
Strict personal hygiene of all staff, whether involved with exposed product or not, is essential in controlling contamination.
Operators should never directly contact exposed product or clean final product containers and caps as they could contaminate the product with skin particles, body oils and hair.
Jewellery should not be worn if the operator has contact with exposed product as it is potentially a source of dirt and grime. Jewellery may also fall into product mixes and remain undetected.
Protective garments should be worn when handling starting materials and exposed product. Once garments become wet or dirty, replace them with a clean, freshly laundered garment.
Wet and dirty garments are a potential source of contamination. Operators and support staff should take particular care when moving between workstations to ensure they are not carrying residual materials on their clothes or footwear.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Conclusion
Contamination control in GMP facility must be taken very seriously as it can jeopardise the quality and safety of food and medicinal products resulting resinous harm to human health.
To control contamination GMP facilities can take measures including but not limited to:
a. Implement strict hygiene practices. Personal protective equipment, such as gowns, gloves, and face masks, should be worn by all employees involved in the production process. Proper hand hygiene should also be followed to reduce the risk of contamination.
b. Use dedicated equipment and facilities. Shared equipment and facilities should be avoided to prevent cross contamination. Dedicated equipment and facilities should be used for each product to ensure that there is no risk of cross contamination.
c. Perform regular cleaning and sanitation. All equipment and facilities should be cleaned and sanitized regularly to prevent the build-up of contaminants. This will help to maintain the quality and safety of the products.
d. Conduct regular quality control testing. Regular analytical and microbiological testing should be carried by the GMP facility to detect and prevent cross contamination. These tests help to identify and quantify contaminants in the product, ensuring that it meets the required standards of quality and safety.
e. Monitor cleanrooms. Contamination control can be accomplished by the use of positive air barriers (to exclude other products), dust extractors and containment hoods (to trap and remove dust) during processing.
f. Monitor material and personnel flow. Design the processing steps in such a manner so that unidentified materials cannot end up into finished products. Implement checks and balances according to Good Manufacturing Practice and regulatory guidelines.
g. Monitor material and personnel flow. Always train your personnel on the procedures that must be followed to avoid contamination in production facilities.
Risks of contamination or cross-contamination arise:
– From starting materials (including water|
– From the environment and from uncontrolled release of dust, gases, vapors, sprays or organisms from materials or products in process
– From residues in equipment
– From operators and their clothing
Contamination control is achieved by:
– Having well-designed and operated facilities
– Good housekeeping practices
– Having written procedures for:
– Handling and storing material and products
– cleaning of equipment and facilities
– Preventive maintenance programs
– Conducting room and line clearances
– Having well-trained operators
– Documenting all deviations or abnormalities

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.