Cleaning validation protocol for pharmaceutical industry
- Kazi
- Last modified: March 13, 2025
A cleaning validation protocol is a detailed plan describing all the validation activities. It comprises a specific validation objective and grouping of products and equipment to validate. will cover.
The protocol should include a risk assessment to identify the worst-case product to clean, as well as the sample locations, sampling plan, types of testing required, acceptance criteria, and test methods you will be using. It should have a section for results, analysis, and final comments.
Cleaning validation establishes that the cleaning procedure can effectively remove product and cleaning residues from pharmaceutical equipment to an acceptable limit.
Cleaning validation verifies that the cleaning procedure can consistently and significantly reduce the amount of active ingredients, excipients, and cleaning agents to a concentration within the acceptance limit.
Calling a piece of equipment clean just because it looks clean is unacceptable in pharmaceutical manufacturing.
Table of Contents
Key Takeaways
- Cleaning validation ensures the efficacy of the cleaning procedure. A validated cleaning procedure produces verifiable evidence that it can effectively reduce residues such as active ingredients, excipients, or cleaning agents to an acceptable limit and prevent them from contaminating subsequent batches.
- Validation of cleaning procedures is a regulatory requirement. USFDA has published a guidance document that sets some expectations for firms to comply with when developing cleaning validation programs.
- A cleaning validation protocol outlines a plan of action. It should include clear objectives, equipment covered, worst-case product risk assessments, testing methods, acceptance criteria, and contingency plans for failed tests.
- Cleaning validation assesses the overall effectiveness of a cleaning procedure. The validation records provide evidence that the procedure effectively removes harmful residues. In comparison, cleaning verification is a routine process to confirm that a cleaning procedure has successfully removed residues from the equipment to an acceptable limit. Cleaning verification is batch specific.
- Risk-based approaches, worst-case products, and scientific justifications are critical when designing cleaning validation protocols. Other considerations include the type of equipment used, solubility and toxicity analysis, cleaning and sanitizing agents, test methods, acceptance limits, sampling plan, swab testing, etc.
- To prove that the cleaning procedure is validated, you need to successfully use it 03 times in a row, either in a campaign or through other acceptable mechanisms.
- Equipment cleaning validation shall be based on a worst-case (indicator) product with the minimum calculated acceptable carryover limit using the calculation model for residue acceptability limit.
- Group the drug products based on similar formulations. At a minimum, indicator drugs for each group should be audited once a year on at least one piece of equipment.
- Equipment can be cleaned manually or in automated (CIP) cleaning processes. To ensure you clean the most challenging spots, you need to find the hardest-to-clean places for swabbing. You might need to swab more than one spot in an equipment.
- Choosing cleaning agents depends on factors such as residue solubility, toxicity, and equipment compatibility.
- Swab and rinse sampling are used to validate cleaning procedures, and the choice depends on equipment accessibility and residue type. You must use validated analytical methods for detecting residues, with acceptance limits based on product toxicity and batch size.
- If an acceptance criterion is not met, initiate a validation deviation, conduct root-cause investigations, and make adjustments where required.
- You should run cleaning validation as a program. The program should ensure each piece of equipment is tested at least once every three years.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Why is cleaning validation required?
Equipment used for processing medicines is one of the top sources of contamination if not cleaned effectively using a validated cleaning procedure.
While manufacturing pharmaceutical products, the equipment’s surface directly interacts with therapeutic active ingredients and many excipients used in the formulation.
Also, the equipment surface is treated with water and various cleaning agents during cleaning and sanitizing.
If even a trace amount of these actives, excipients, or cleaning agents is not removed properly during the cleaning process, it may cause harmful contamination of the next product.
A pharmaceutical manufacturing plant compliant with Good Manufacturing Practices (cGMP) must have a cleaning validation program to establish documented evidence that the cleaning processes will consistently meet expectations by removing traces of residues from the earlier products.
Doing so ensures the purity, safety, and quality of the next products in the campaign are not compromised.
Cleaning validation ensures that no significant amounts of active ingredients or excipients are carried over into subsequent equipment uses.
What is a cleaning validation protocol?
A cleaning validation protocol can guide you through the entire validation process.
Before starting any validation activity, you need to devise a plan. Preparing a cleaning validation protocol is the first step in planning.
Typically, a protocol includes a clear and specific validation objective, followed by the collection or grouping of equipment that the protocol will cover.
To begin with, the protocol must address the description of product or formula attributes.
A cleaning validation risk assessment should be included as part of the protocol to identify the worst-case product based on the therapeutic ingredients and excipients and their solubility and toxicity profiles.
A cleaning validation protocol should include the types of testing required, acceptance criteria, sample locations, sampling plan and procedures (e.g., swab or rinse samples), analytical and microbiological test methods to be used, etc.
The validation protocol also has instructions for the cleaning sequence, cleaning agents, and cleaning period.
The validation protocol also includes calculating the equipment train surface area, acceptance limits, and a procedure for recovery studies.
It should include a list of cleaning equipment necessary for carrying out the protocol and safety instructions on using chemicals and sharp equipment.
Despite the careful planning process, there is always a chance the validation tests may need to meet the acceptance criteria. The protocol should have guidelines on how to manage failed results.
If you have to amend the protocol to include a correction derived from an investigation, please utilize the protocol change management approach.
What should be included in a validation protocol template?
If you are preparing a cleaning validation protocol for processing equipment, you should include the following essential steps:
– Clearly describe the cleaning validation objectives.
– Provide the background and scope of the cleaning validation.
– Approval of the protocol by the authorized personnel and SMEs with signatures and date approval.
– Approved cleaning procedure and records needed during validation.
– Products to be addressed by the protocol.
– Risk-based analysis to determine the worst-case product as the benchmark.
– Description of equipment and cleaning cycle.
– Reference to the qualification of all major equipment involved.
– Product residue detection.
– Residue materials to be removed.
– Microbial bioburden.
– Detergent removal process.
– Maximum clean and dirty hold time.
– Sampling plan
– Campaign lengths, if applicable.
– Cleaning parameters to be evaluated.
– Cleaning and sanitizing materials to be used.
– Identification of analytical test methods to be used for testing.
– List of microbiological test methods.
– Describe sampling plans and methods.
– Surface area calculations.
– Procedure for recovery studies.
– List of acceptance criteria with calculations.
– List of cleaning instruments that will be required.
– Validation deviation handling process.
– Protocol change management, etc.
You can follow the link cleaning validation protocol sample to develop one.
This cleaning validation guideline also presents the approach and methods for validating cleaning and sanitation procedures for your facilities.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
What is FDA guidance on cleaning validation?
USFDA has published a guidance document, “Guide to Inspections Validation of Cleaning Processes” where it has set some expectations for the firms to comply with when developing cleaning validation programs.
For example, the FDA expects firms:
– To have written procedures (SOPs) detailing the cleaning processes used for various pieces of equipment.
– To have written general procedures on how cleaning processes will be validated.
– The general validation procedures to address who is responsible for performing and approving the validation study, the acceptance criteria, and when revalidation will be required.
– Prepare specific written cleaning validation protocols in advance for the studies to be performed on each manufacturing system or piece of equipment. These protocols should address issues such as sampling procedures and analytical methods, including their sensitivity.
– To conduct the validation studies in accordance with the protocols and to document the results of studies.
– A final validation report approved by management states whether the cleaning process is valid. The data should support the conclusion that residues have been reduced to an “acceptable level.”
Difference between cleaning validation and cleaning verification
Cleaning validation is a systematic process to demonstrate that a cleaning procedure consistently and effectively removes residues such as active ingredients, excipients, and cleaning agents to ensure product quality in future batches is not compromised.
Cleaning validation covers the entire cleaning process, from selecting cleaning agents, sampling procedures, setting up acceptance criteria, testing, and recording.
It is typically performed before introducing a new product into production or when significant changes are made to the cleaning process.
Cleaning validation is not a routine procedure but is conducted as needed or when specific circumstances warrant validation, such as product changeovers or equipment upgrades.
A successful cleaning validation results in a validated cleaning procedure, which is documented and used as a standard operating procedure (SOP) for routine cleaning.
In contrast, cleaning verification is a routine process to confirm that a cleaning procedure has successfully removed residues from the equipment to an acceptable limit.
Unlike cleaning validation, cleaning verification is not concerned with the overall effectiveness of the entire cleaning procedure but rather focuses on confirming the cleanliness of a specific batch.
Cleaning verification is typically performed by the end of each cleaning process to ensure that a particular batch has met cleanliness criteria.
Things to consider for cleaning validation
A cleaning validation project is a delicate operation. You have to follow a very specific methodology during cleaning validation activities.
While implementing a cleaning validation program, there are several key considerations you need to be aware of.
However, remember that the types of equipment and their utilization in the manufacturing process have endless possibilities.
Hence, one set of considerations may not fit for all circumstances.
a. You must validate all cleaning procedures for all stages of the manufacturing process where there is a chance for cross-contamination.
b. You can exclude any equipment from the validation project that does not come with product contact.
c. Establish a mechanism to ensure that only validated equipment and facilities are allowed to manufacture pharmaceutical products. If you must use the equipment, ensure visual inspection is completed and analytical results are available until the cleaning process is validated.
d. A cleaning procedure must be in place before you can initiate cleaning validation for any product, equipment, or facility. At a minimum, the cleaning procedure should contain the following information:
– The quantity and concentration of the cleaning solution are to be used.
– Type of cleaning agents to be used.
– Methods of using the cleaning solution.
– Exposure time of cleaning agent to surface.
– Rinsing process, and
– Specify the time between the product run and the point of cleaning.
e. To prove that the cleaning procedure is validated, you must successfully run the process 03 times in a row. But if you are cleaning a different product using different cleaning procedures in between those 03 times, you have a few options:
i. You can do the cleaning validation tests at the end of the scheduled campaign. And/or,
ii. You can perform the cleaning validation tests at cleaning campaign lengths shorter than the maximum campaign length as long as at least one test is performed at full length.
iii. If needed, you can also do extra tests with a product that’s even harder to clean before finishing the three tests for the toughest product.
f. For retrospective cleaning validation studies, you should consider the second hardest-to-clean product if the toughest one is rarely made. Failing a validation test for the harder-to-clean product also counts as failing for the toughest one.
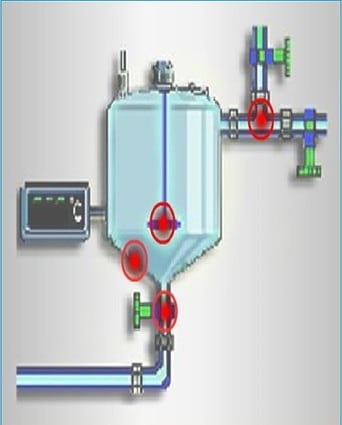
g. To ensure you clean the most challenging spots, you need to find the hardest-to-clean places for swabbing. If you think contamination is evenly spread, you have to figure out these tough spots.
In your cleaning validation protocol, you must specify where you’ll swab, especially if you might need to swab more than one spot on the equipment.
h. You should initiate cleaning validation whenever a new drug product is introduced into multi-purpose production equipment.
Quarantine the equipment for appropriate assessment. Consider the cleaning validation part of the new product’s production scale-up.
i. Adopt a risk-based approach to cleaning validation, focusing on critical areas and worst-case scenarios. Prioritize resources and efforts based on the risk associated with each piece of equipment.
j. Whenever feasible, utilize an indicator drug product for cleaning validation. The indicator concept allows products to be grouped based on similar formulations, similar active ingredients and shared production equipment.
k. Equipment cleaning validation shall be based on a worst-case (indicator) product with the minimum calculated acceptable carryover limit using the calculation model for residue acceptability limit.
l. The residue acceptability limit should be based on the toxicity limit for non-therapeutic drug products. Use the minimum calculated acceptable carryover limit as the cleaning validation criteria for all products manufactured in the specified equipment.
m. After the cleaning validation, establish a periodic monitoring or audit program for all marketed products.
n. Group the drug products based on similar formulations. Indicator drugs for each group should be audited at least once a year on at least one piece of equipment. The program should ensure each manufacturing equipment is tested at least once every three years.
o. Validate the effectiveness of cleaning agents to ensure their ability to remove residues. Consider factors such as material compatibility with the equipment, residue solubility, and environmental impact.
p. Dedicated equipment in manufacturing operations will require cleaning. The cleaning procedure must specify the method and frequency of cleaning.
The cleaning procedure and the cleaning log for dedicated equipment must provide for verification of removal of all visible product residue after each clean-up.
Cleaning validation is not required if cleaning verification is performed after each clean-up.
q. Select appropriate analytical methods and sampling plans to measure and detect residues accurately. The methods should be validated, and the sampling plan should consider worst-case scenarios and representative sampling locations.
r. Establish scientifically justified acceptance criteria based on product safety, potency, and regulatory requirements. Consider factors such as toxicity, pharmacological activity, and manufacturing process.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Types of cleaning processes and cleaning agents
Before planning for cleaning validation, it is important to understand the different types of cleaning procedures and cleaning agents used.
a. Manual cleaning process
Several nondedicated equipment, such as mixing vessels, granulators, blenders, dryers, etc., can be cleaned manually. These are smaller in size and easily portable between processing areas.
Tablet compression or filling machines, homogenizers, fluid bed dryers, etc., are also cleaned using manual cleaning procedures.
Purified and distilled water is primarily used for cleaning where the active and excipients are soluble. Other cleaning agents are also used if the residues are partly or fully insoluble in water.
The selection of a cleaning or sanitizing agent is dependent upon:
– The previous product type, for example, a cream or powder.
– Compatibility with equipment to be cleaned.
– Ease and safety of use.
– Ease of removal of residues.
– Required contact times on equipment.
– The targeted microbial population.
b. Automatic cleaning in place (CIP)
Large processing equipment, such as sterilization tanks, mixing tanks, storage vessels, etc., can be equipped with an automatic cleaning-in-place (CIP) facility.
CIP is the procedure by which flush and rinse solutions are sprayed onto all internal product surfaces without the intervention of operators and continuously re-circulated or flushed to drain for a pre-determined time.
CIP systems rely on chemical removal and physical agitation of the pipes, valves, and tanks rather than just physical removal of residues.
The success of CIP systems is determined by their design, installation, and commissioning/validation.
The design must ensure no “dead legs” (areas where cleaning solution cannot penetrate) and facilitate the complete rinsing of any residues.
The CIP system may be a “recirculating” or “once-through” design. Both systems must be controlled and alarmed to detect malfunctions, and the system is only considered clean after a successful cleaning cycle.
As with manual equipment cleaning, CIP systems must be validated to ensure that the previous product has been removed, no residual cleaning agent is left after cleaning, and the microbial population has been reduced to a safe and acceptable level.
The SOP for an automated cleaning procedure does not need to state precisely how the equipment is cleaned.
However, cleaning validation must document and verify that the automated process operates according to its design requirements.
Cleaning in place (CIP) operating criteria
i. Most CIP applications will use fresh distilled water discharged through fixed spray balls, providing complete coverage of the inside of vessels or discharged directly into product transfer pipework via change piece.
ii. The CIP flow velocity, temperature, and required cleaning time must be verified and documented for each cleaning validation test. Where CIP flow switches are installed, it is important to verify that correct alarm operations are included.
c. Cleaning agents
Cleaning agents such as detergents and other chemical aids should only be used when water or solvents are not adequate. This may be due to insoluble materials, difficult-to-clean equipment, or other environmental factors.
Only approved cleaning agents whose compositions are known should be used. This allows analytical measurement of residues and has proven to be easily removable.
The following is a list of commonly used sanitizers. However, you must validate their condition and efficacy by conducting trials.
Always remember to include only approved sanitizers in your cleaning procedure.
– 70% v/v alcohol (for surfaces).
– Quaternary ammonium compounds (QUATs).
– Phenolics.
– Sporicides (for killing spores).
– Chlorines (such as hypochlorite).
– Hydrogen peroxide/peracetic acids.
– Sodium hydroxide (cleaning & partial sanitizing).
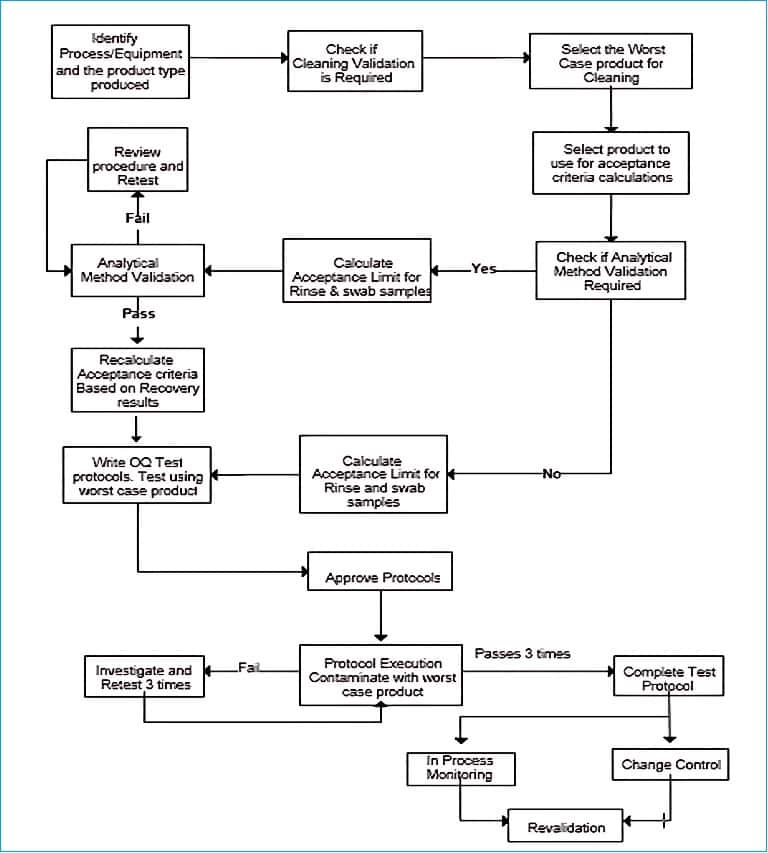
How to perform cleaning validation step-by-step?
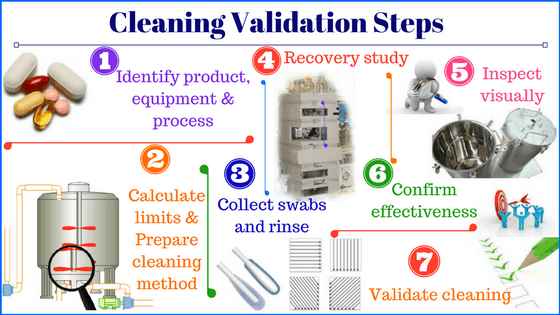
1. Identify process, equipment, and product type
Identify the process and the types of products you produce. List the types of dedicated and shared equipment used for processing those products.
Perform a risk assessment to identify an indicator drug product. The indicator concept allows products to be grouped based on similar formulations, similar active ingredients, and shared production equipment.
Could you identify the worst-case product based on your assessment of solubility rating and toxicity?
Include these details in the validation plan and the validation protocol.
2. Check if cleaning validation is required
As a rule of thumb, if your process or equipment falls into any of the following types a cleaning validation assessment is required:
– Holding vessel
– Mixing vessel
– Product transfer Line
– Other equipment that performs an automated cleaning of product contact parts.
3. Cleaning validation for new processes and equipment
If introducing a new process and equipment into your facility, consider full validation testing in most cases. This involves three tests of worst-case products with the appropriate number of samples collected.
Reduced testing is feasible if all the following conditions are met:
i. Identical process or equipment is installed.
ii. Identical cleaning controls and procedures are achievable.
iii. Worst-case product is the same as previously validated and acceptance criteria is the same or higher than previously validated.
iv. Cleaning validation data is available on the previously validated identical process.
Could you explain reduced testing in the relevant validation Plan and protocol? Reduced testing may include reducing the number of tests and sample locations to a few key critical areas known as “critical sites” or “hot spots.”
For changes to current cleaning processes and procedures, the extent of retesting may be reduced to a single test. This should be stated and approved as part of the change request process, if you don’t mind.
4. Select the worst-case product for cleaning validation
For multi-product equipment, it is not practical to validate the cleaning of all products with one cleaning process where products are alike in formulation and dosage form.
In such cases, you will benefit by selecting a worst-case (indicator) product to represent all products in the cleaning validation process.
The worst-case selection is based on a scientific justification: the least soluble product produced on a particular piece of equipment or process is then used to validate cleaning for that piece of equipment or process.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
5. Acceptance criteria calculations for worst-case product
The calculation of acceptance criteria is to be based on the most toxic product within a group of products produced in each process.
Find the product within the product group with the lowest active toxicity value in mg/kg; this is the most toxic product. The lowest active toxicity value will be used to calculate acceptance criteria.
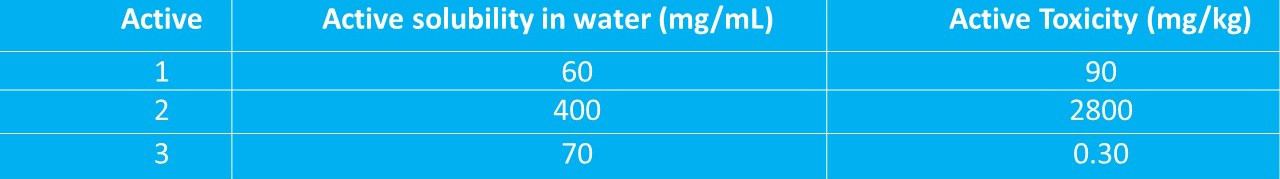
Example of selecting worst-case product and product for acceptance criteria calculations
Note – a lower toxicity value means the substance is more toxic, a higher value means lower in toxicity.
From the above table of product data, Active 1 is the least soluble product in water since it has a value of 60 mg/mL and is therefore the worst-case product in this group.
Active 3 is the most toxic product since it has the lowest toxicity value of 0.3 mg/kg. Therefore, the acceptance criteria are calculated using active 3 product data.
6. Cleaning validation for new product introduction
If a new product is introduced into the facility the solubility and toxicity of the new product should be compared against the current list of products in the same product type group and the following assessment should be made:
– If the new product is less soluble than the current least soluble product within the product group then this product becomes the new worst-case product for that process. Cleaning validation, including analytical method validation, should be conducted for the new product.
– If the new product is more toxic than the most toxic product within the group then the worst-case product does not change but the acceptance criteria to be applied to the worst-case product must be recalculated according to the product data for the new product. If previous validation data shows the results meet the new acceptance criteria no further cleaning validation is required.
– If the solubility of the new product is not less and the toxicity not higher than the current listed products then no cleaning validation is required for the new product.
7. Select the sampling technique for cleaning validation
Sampling for cleaning validation will involve rinse water and swab techniques.
You should use swabs for difficult-to-clean areas and when contact surfaces are physically accessible.
Surfaces inaccessible to swab samples such as transfer pipes should be sampled using a pre-determined volume of final rinse solution, usually water.
Include the preferred sampling technique in the cleaning validation Protocol. The technique must indicate the sample materials required, the sample solution (e.g., water type), the rinse volume or swab sample areas, and the sample locations.
i. Swab sampling
The swab method should be based on the procedure validated by the analytical laboratory. In many cases, the surface of production equipment will not be a flat stainless steel surface. Therefore, the swab must be done as close as practically possible to the validated swab procedure.
Non-standard swab areas
Where it is not possible to swab 100cm2 you should record the actual area used for the swab and make an adjustment to the acceptance limit. For example, if the swab area is only 50cm2 the acceptance limit should be halved.
ii. Rinse sampling
The rinse method should be based on the procedure validated by the Analytical laboratory.
Non-standard rinse volumes
Where it is not possible to rinse to the required ratio of Rinse to the Surface area, record the actual volume, and make an adjustment to the acceptance limit. For example, if the rinse volume calculated is 1L and 2L is required, the acceptance limit should be halved.
8. Validate analytical method for cleaning validation
After selecting the worst-case product, check if an analytical method is validated. If it is not, analytical method validation is required.
You can follow the step-by-step process to complete an analytical method validation.
i. Active residue analytical testing
This test is conducted to determine the accuracy of measuring the active at concentrations above and below the calculated acceptance criteria levels for cleaning. This is done simply by preparing the relevant concentrations in volumetric flasks and analyzing the samples using Chromatographic procedures or Total Organic Carbon (TOC).
This tests the accuracy and precision of the analytical method without any effects of sampling recovery from surfaces by swabbing or rinsing.
ii. Rinsing recovery studies
Rinse recovery studies must be conducted to test the specific product on the production equipment. This must be completed before rinse samples can be taken from equipment surfaces. This will ensure that the product can be recovered from the equipment surface with an adequate recovery level.
Method validation studies should determine the repeatability, reproducibility, and recovery of the rinsing analysis from the equipment surfaces.
If recovery results do not meet the acceptance criteria, a different solvent or a larger rinsing volume may need to be used.
iii. Rinsing procedure
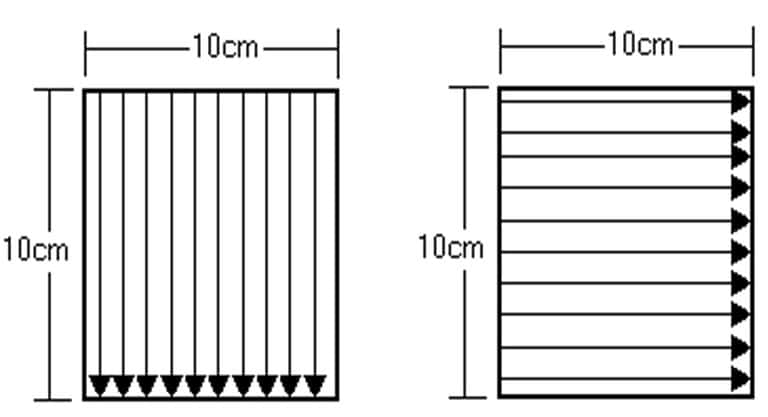
Rinse recovery studies are performed by spiking 316L stainless steel plates (or other production material if more relevant for a given process). The product is evenly distributed onto the plate at concentrations above and below the acceptance criteria calculated in step “i. Calculate acceptance limits for rinse and swab samples.”
The stainless-steel plates must be large enough to allow a 10 cm x 10 cm surface area to be rinsed. Allow the product to dry on the sample surface before rinsing. Rinse the plate with a specified and accurate amount of water i.e. using a pipette.
Collect the rinse water into a beaker and analyze the rinsate according to the analytical procedure using High-Performance Liquid Chromatography or Total Organic Carbon (TOC).
TOC samples should always be analyzed as soon as possible after sample collection. i.e., within 12 hours.
iv. How to collect rinse samples?
In some cases, both chemical and microbiological samples are required for each rinse sample. If this is the case, collect the microbiological sample first. Then, aseptically transfer some of the solution into a sample container for chemical testing.
You’ll need to make sure you maintain enough precautions for manual rinse samples to avoid compromised collected samples.
Containers for collecting samples, such as sample jars, trays, buckets, etc., must be cleaned and thoroughly rinsed with distilled water, especially when conducting conductivity measurements and conducting TOC analysis.
For TOC testing, use clean TOC vials or glass Schott bottles. Devices such as manual valves used to collect samples must be cleanable and always cleaned prior to use.
Containers that pressure transfer water samples through product lines must also be cleaned and rinsed thoroughly with Distilled water.
For TOC testing, it is important to collect a small sample of the rinse water used as a blank sample to measure the background of TOC.
The main requirement for collecting microbiological samples (bioburden) is that sample containers be pre-sterilized. The sample valves are clean and pre-sanitized by flushing with 80°C distilled water for 5 minutes.
v. Swabbing recovery studies
Swab recovery studies must be conducted to test the specific product on the production equipment.
This must be completed before swab samples can be taken from equipment surfaces. This will ensure that the product is adequately recovered from the equipment surface based on the appropriate selection of swab material and solvent.
Method validation studies shall determine the repeatability, reproducibility, and recovery of the swabbing analysis from the equipment surfaces.
If recovery results do not meet the acceptance criteria, a different swab type, solvent, or swabbing method may need to be used.
vi. Swabbing procedure
Swab recovery studies are performed by spiking 316L stainless steel plates (or other production material if more relevant for a particular process). The product is evenly distributed onto the plate at concentrations above and below the acceptance criteria calculated in step “i. Calculate acceptance limits for rinse and swab samples.”
The stainless-steel plates must be large enough to swab a 10cm x 10cm (100cm) surface area. Allow the product to dry on the sample surface before swabbing.
Following is a recommended procedure to follow, which has been shown to work well for Method Validation tests:
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Place 2 swabs into 1 clean 40mL TOC vial containing the required volume of extraction solvent (usually 25mL).
The 2 swabs will be used for the same 10 x 10cm surface and placed back into a single vial.
The swab surface is pressed on the side of the glassware to express excess water before use. The swabbing is conducted by covering the area in one direction and then using the flip side of the swab to surface swab in a perpendicular direction. Firmly press down on the swab handles to ensure proper surface contact. The second swab is removed from the solvent, and the first swab is placed back into the solvent.
The procedure is repeated with the second swab, ensuring that the same 10cm x 10cm area is swabbed. The swab is placed back into the solvent.
The swab handles are cut with a clean pair of scissors, ensuring no foreign particles are introduced into the solution. The solution is vortexed for 30 to 60 seconds.
TOC samples should be analyzed as soon as possible after sample collection, i.e., within 12 hours.
vii. How to collect swab samples?
The principles explained under rinse samples also apply to swab sampling. The swabbing procedure must be based on the procedure validated as part of the analytical method validation.
The relevant file for method validation should be used to describe the swabbing procedure in the test protocol.
viii. Why do you need two swabs for method validation?
You should use two swabs to increase recovery from equipment surfaces. The first swab may collect 70% of the residue.
The second clean swab can absorb the remainder more easily from the surface. Also, when two swabs are placed inside the 40mL TOC sample vial, this provides a greater mixing action than 1 swab when the samples are vortexed.
ix. Selection of analytical testing instruments
Method validation should be performed for most applications using TOC and HPLC analysis.
However, if the product to be validated has no additional excipients containing organic carbon other than the active ingredient, then only HPLC needs to be used.
If, however, it is necessary to have the flexibility of using either TOC or HPLC analysis, then both analytical tests must be validated. The detailed analytical test method used for quantifying the results must be recorded in the analytical method validation files.
9. Calculate acceptance limits for rinse and swab samples
i. Basis of cleaning validation calculation
To calculate the acceptance limits, use the lowest concentration of the most toxic active in a product group, combined with a safety factor of 1/1000th.
Once an agreed acceptance limit is established, it is applied to an area/weight limit based on factors such as batch size and calculated surface area of product contact.
The final calculated limits are then applied to the worst-case product in a product group.
ii. Establishment of acceptance criteria
Calculating acceptance criteria for rinse and swab samples gives you two options. You should apply the most stringent of the two.
Option 1: Absence of visible residues and Maximum Allowable Carryover (MACO).
Option 2: Any subsequent batch will not contain visible residues or more than 10ppm of carryover of the previous product.
Therefore, option 2 is applied when the calculated Maximum Allowable Carryover (MACO) is above 10ppm OR if there is no clinical data, such as the lowest concentration dose for the residue to be calculated.
iii. What are MACO and NOEL in cleaning validation?
MACO stands for Maximum Allowable Carryover limit in cleaning validation. MACO represents the maximum residue level permitted from one completed batch to the next after cleaning the multi-use equipment. The amount of residue if present is assessed to have no harmful effect on human use.
NOEL stands for No Observable Effect Level in cleaning validation. It defines the highest residue level with no discernible adverse effects. This is the limit at which no effects on the subsequent product or recipient can be detected.
NOEL is particularly important when dealing with highly potent drugs.
How to calculate Maximum Allowable Carry Over (MACO)
When batch and single active concentration volumes for multi-product equipment are known, the total MACO (in the entire processing system) is calculated as follows:
MACO = (LC) x (SBS)
(SF) x (LVSD)
MACO = maximum allowable carry-over
LC = lowest concentration (in mg)
SBS = smallest batch size (in ml) made in the same equipment
LVSC= largest volume single concentration (active) of any product made in the same equipment (in ml)
SF = safety factor = 1000. This is based on biological activity levels of 1/1000 of the normal active concentration.
Example of Maximum Allowable carry-over calculation
MACO = [0.25mg x 200,000mL] ÷1000 x 2.0mL = 25.0 mg
Therefore, the total quantity of residual product allowable in a subsequent production batch is 25.0 mg.
iii. Calculating acceptance limits for swabs
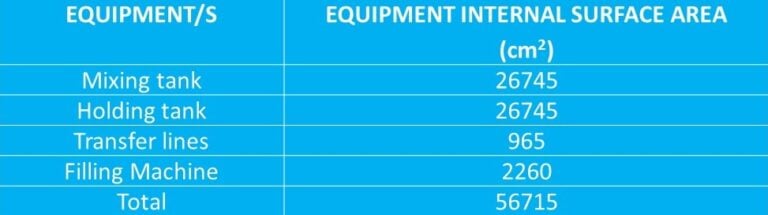
Take the calculated MACO for the product and divide this number by the total internal surface area of the total product processing system, i.e., preparation and holding vessels + pipework + filling machine.
This figure is the amount of residue allowed throughout the entire process, assuming that there is even distribution of product residue throughout the process equipment.
Example calculation:
The allowed residue in the entire process = MACO ÷ total surface area
= 25.0mg ÷ 56715 cm2
= 0.00044 mg/cm2 (MACO/cm2)
The overall surface area is derived from the following (only as an example):
This calculated value determines the amount of residual product allowed to remain on 1 square centimeter of the equipment after cleaning. This value is then multiplied by the area to be swabbed to give the allowed limit per swab sample.
If swabbing a 10 cm x 10 cm (100 cm2) surface area and placing the swab in 25.0ml of the swabbing solution, then the following should apply:
Limit for swab sample = [MACO/cm2 x Swab area] ÷ The volume of Swab Solution
= [0.00044 mg/cm2 x 100 cm2] ÷ 25.0 mL
= 0.0088 mg/mL (8.8mg/mL or ppm per swab)
In this case, swab sample results for 100 cm2 must be < 8.8 mg/mL of active to prove satisfactory cleaning.
The 0.00044 mg/cm2 value was derived from the MACO allowed per swab calculation above.
iv. Calculating acceptance limits for rinse samples
a. Calculate the required rinse volume
Rinse recovery method validation tests are conducted with a particular volume of solution per surface area of the test material.
After determining the ratio of rinse volume to surface area, apply this to the area of the sample process.
For example, rinse recovery method validation records the ratio of rinse to surface area to be 20mL/100 cm2; for a pipe of surface area 2500 cm2, the required volume is 20/100 X 2500 = 500mL (0.5L).
b. Calculating Limits for rinse samples
To calculate, take the MACO/cm2 derived earlier and multiply it by the total internal surface area of the processing system.
Then, divide the result by the rinse sample volume.
For example, using the MACO/cm2 calculated above, rinse sample limits are calculated as follows:
Limit for rinse sample =
[(MACO/cm2) x Pipe or Vessel Surface Area *] ÷ Rinse Water Volume.
= [0.00044 mg/cm2 x 965 cm2] ÷ 500 mL
= 0.00085 mg/mL or 0.85 ppm
*actual surface area of the specific pipe or vessel being rinsed.
In this example, a 500 mL rinse water sample of the transfer line, with a surface area of 965 cm2, must be < 0.85 ppm of residual active to prove the cleaning process is satisfactory.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
10. Limit of detection (LOD) and Limit of quantitation (LOQ) check
After you calculate the final swab and rinse limits, please check that the limit of quantitation (LOQ) for the worst-case product is below the calculated acceptance criteria.
If accurate analysis is restricted by the limit of quantitation (LOQ), the limit of detection (LOD) can provide a pass/fail result.
For example, if the LOQ is 700 parts per billion (ppb) and the LOD is 200 ppb, for a swab sample with a failed result of 190 ppb, the laboratory would record a “FAIL” rather than a numeric value.
11. If samples fail to meet acceptance criteria
You must investigate if a rinse or swab sample failed to meet the pre-determined acceptance criteria during cleaning validation. After the investigation, you can change procedures and then repeat the tests.
Sampling, testing, re-sampling, and re-testing the same equipment should not be conducted if test results continually fail to meet the Acceptance Criteria.
The “test until clean approach” or testing until the desired results are obtained demonstrates the cleaning process is not in control. If this occurs, the cleaning procedure must be investigated for root cause. This may include,
– Alteration of product contact materials such as flexible transfer tubing.
– Improvement of the cleaning cycle.
– Extending the flushing time.
– Removing potential dead legs, etc
Conclusion
Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives, excipients, and cleaning agents, and maintain product quality in future batches.
A cleaning validation protocol is a comprehensive plan that guides the entire validation process. It includes clear objectives, equipment and product details, worst-case assessments, sampling procedures, testing methods, acceptance criteria, safety instructions, and protocols for managing failures or changes.
The FDA provides guidance on cleaning validation, emphasizing the importance of written procedures, validation protocols, and final reports to demonstrate the validity of the cleaning process.
Key considerations for cleaning validation also include a risk-based approach, using indicator drugs, using approved cleaning agents, validated analytical methods, and periodic monitoring.
Cleaning validation is not just a regulatory requirement; it is a critical program that ensures the integrity and safety of your products.
By implementing the cleaning validation strategies and practices outlined in this guide, you can minimize the risks associated with cross-contamination, product quality defects, and regulatory non-compliance.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.


1mg/mL = 1000ppm. It is not a one to one ratio. 1mg/L is 1ppm. You may want to issue a correction.
Do we able to manufacture and production for pharma product before getting the Api and micro swab result ? What will be the risk ?
Do we able to manufacturer and filling before getting the validation swab result?
How do I measure the surface area of a particular machine part
Hello, inspectors ask how we calculated surface area of equipment that we validate. For some equipment we have exactly data for surface in specification. But fore some parts of equipment we used calculation based on aproximation in line to known geometrical shapes. Inspector sad that we need to have that calculation recorded somewhere and show it on request. Please if you have some advice to share. Thank s in advance.
How many batches are required for cleaning validation
A minimum of three batches of the same API/s and detergent solution should be selected to produce statiscally sigfnificant validation evidence.
Is there any relationship between sore of products and cleaning validation
Nice article!