Control of microorganisms in pharmaceuticals production
- Published on: Dec 16, 2020
Micro-organisms are mostly killed by exposure to heat above 55 – 60°C for periods of time. The heat breaks down the cell wall, which kills the micro-organism. The cell walls of spores, however, are resistant to anything but high heat.
Similarly, alcohol is a good general sanitizing agent, but there are some micro-organisms that are not killed by alcohol (such as spores).
A combination of heat and alcohol provides two methods of sanitizing. Of course, sanitation will only be effective if a validated procedure is followed. Pay particular attention to:
- Pre-cleaning to remove most of the chemical residues and bio burden
- The length of time the heat and sanitizing agents are applied for: too short an exposure time will be less effective.
- Product degradant on, making products ineffective
- Consumer safety, as products could become health risks
- Use of the product (topical or oral)
- Nature of the product (supports/inhibits growth)
- Potential hazard to the user (children/sick/old)
- It would be virtually impossible to completely validate test procedures for every organism that may be objectionable. However, it is a good practice to assure that inhibitory substances in samples are neutralized.
- For products such as topicals, inhalants, or nasal solutions where there is a major concern for microbiological contamination, isolates from plate counts, as well as enrichment testing, should be identified.
- It is recognized that pseudomonads are objectionable if found in a topical product or nasal solution in any numbers. There are no test methods provided in the USP that will enable the identification of the presence of this micro-organism.
- Control the manufacturing environment
- Wear protective clothing
- Implement cleaning and sanitation programs
- Keep equipment clean and dry
- Maintain room pressures by keeping doors closed
- Maintain a high standard of personal hygiene
- Starting materials control
- Production equipment control
- Water and other critical services control
- Environmental control
- Personnel control
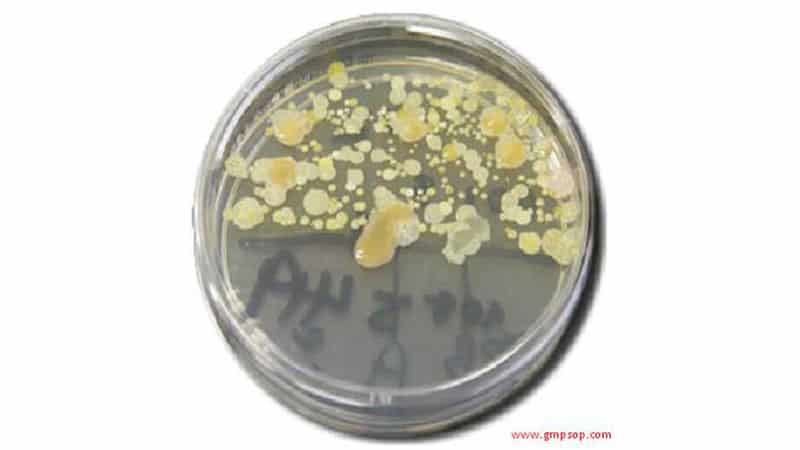
- Microbiological testing is far from absolute.
- Negative test results may merely indicate insensitivity of the test.
- Microbiological contamination is not evenly dispersed throughout a lot or sample of product.
- Finding a contaminant in one sample and not in another does not discount the findings of the initial sample results.
- Other organisms may mask the presence of certain organisms when identification procedures are performed.
- Store starting materials clean, dry, and if necessary, cold.
- Store starting materials off the ground, and under cover.
- Always keep containers sealed when not in use.
- Be alert to damaged packages.
- Never use dirty or wet sampling or measuring equipment.
- For topicals and inhalants, there must be no presence of pseudomonads in the water testing profile.
- Systems must be able to be regularly sanitised. Each unit within a water system may have different sanitation conditions.
- Manufacturers need active change control and maintenance programs.
- The QC test program should carefully document:
- Sample points and timing “worst-case”
- Sufficient frequency of testing in order to generate meaningful results
- Pay attention to the limits and responses to out-of-limits.
- Air handling systems
- Designated manufacturing zones
- Cleaning programs
- Staff wear protective garments (including caps and shoes) that are clean, and are only worn in designated processing areas.
- Product isn’t handled with bare hands.
- GMP induction and ongoing GMP training include personal hygiene and sanitation rules.
- Personnel wash hands after visiting the toilet.
- Micro-organisms make up more than 50% of the weight of human faeces (approximately 1 trillion/g). Even after washing hands, your skin will again contain over 20,000 microbes per cm2.
- Airlocks between toilets and production areas
- Any staff infections are reported to supervisors
- There are policies in place prohibiting food, cosmetics and jewelry in production areas.
- Micro-organisms are very adaptable, and are found everywhere.
- Micro-organisms need only small amounts of nutrients and moisture to grow, and they can grow very quickly.
- Objectionable organisms should not be present in a product because they can reduce the product’s effectiveness and cause possible harm to consumers.
- Although micro-organisms cannot be necessarily eliminated entirely, manufacturers need to keep micro-organisms at acceptable levels.
- To reduce the numbers of micro-organisms in the workplace, there must be strict controls in place over the manufacturing environment, starting materials, equipment, pharmaceutical water, the environment, and personal hygiene.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
You may like to read these related posts.
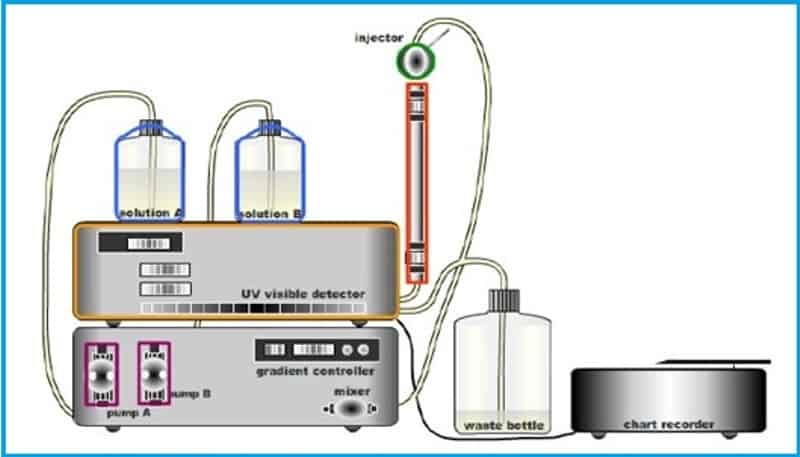
Chromatographic systems used in Pharmaceutical Laboratories
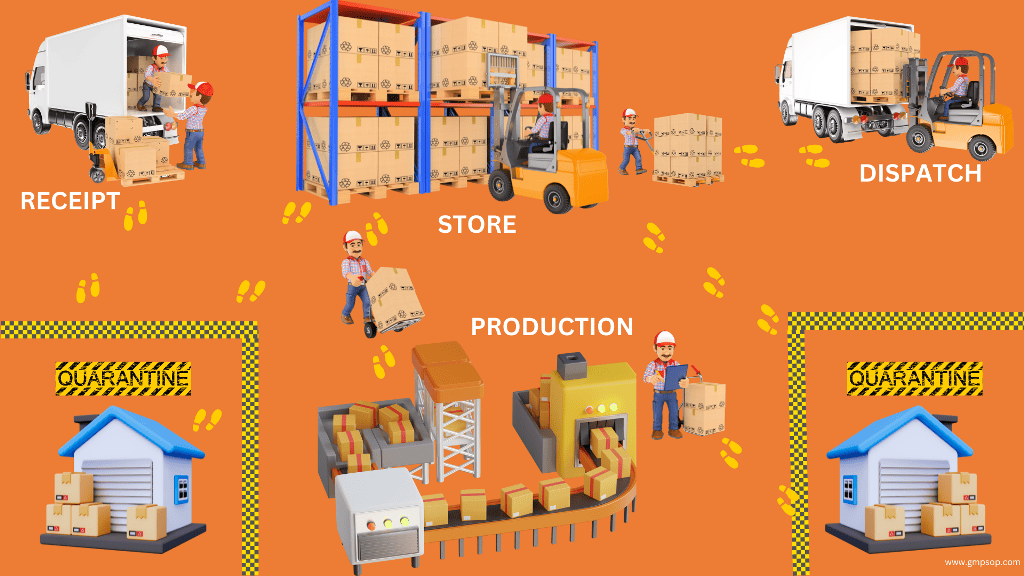
Warehouse material handling for pharmaceutical industry
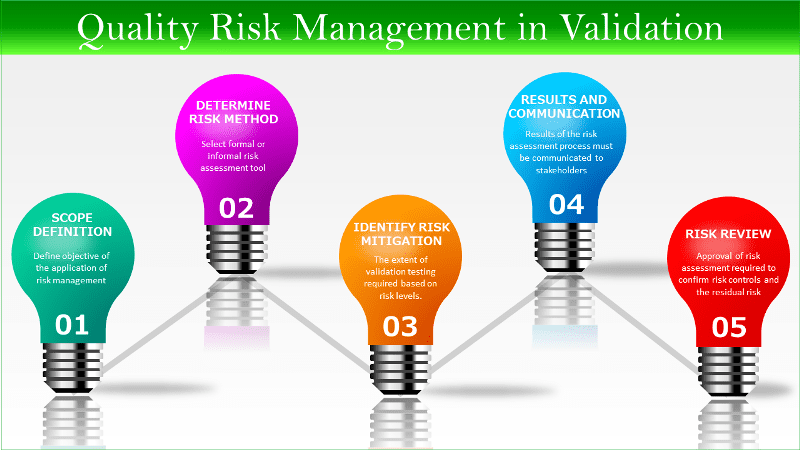
How to use quality risk management in validation testing