Corrective and Preventive Action (CAPA) Procedure for GMP
- Kazi
- Last modified: March 23, 2025
If you are looking for literary help to write an actionable procedure on corrective and preventive action for your workplace or school, this article will be a good place to start.
Consider this scenario. You are working as a Laboratory Manager and one of the chemical tests was reported to be out of specification. The test result fell outside the specified range. What would you do?
Will you ask the Analyst to redo the same test with a hope that this time the result will pass? No, this will be a violation of good laboratory practice.
Ideally, you should halt further testing. keep the samples and instruments in their last original positions. Immediately communicate the incident to stakeholders and initiate an internal laboratory investigation.
The objective of the laboratory investigation is to detect if the error was contributed by laboratory environment and/or sampling error.
To do that, you should interview the Analysts if they have followed the correct analytical method. Review Analyst’s laboratory workbook. Check if the test method was validated and approved. Check the calculation, instrument calibration status, sample measurement technique, sampling procedure or sample handling, etc.
This is done to rule out if the laboratory conditions or sample had inadvertently contributed to the out-of-specification result.
If it was found the laboratory conditions or sample have directly caused the incorrect result, you should correct the issues and redo the testing.
However, if it is proven that the laboratory conditions or the sample were not to blame, beyond a reasonable doubt, the result will be confirmed as out-of-specification. In that case, you must raise a deviation and call the other stakeholders for a detail investigation.
Once the deviation investigation will complete, you will likely find the root cause of the incident. You should take corrective and preventive actions as directed by the outcome of the investigation.
You may have to resample and retest as part of the investigation. If the new result appears out-of-specification again, you may have to reject the material to avoid compromising the quality of your product.
Corrective actions only manage the issues on hand. To stop the recurrence of similar incidents you need to take preventive measures.
You may have to qualify a new supplier, re-train the employees on operating procedures, re-validate the method to make it robust, or improve storage conditions, etc.
Basically, you should take all sorts of preventive measures as directed by the outcomes of the investigation.
Table of Contents
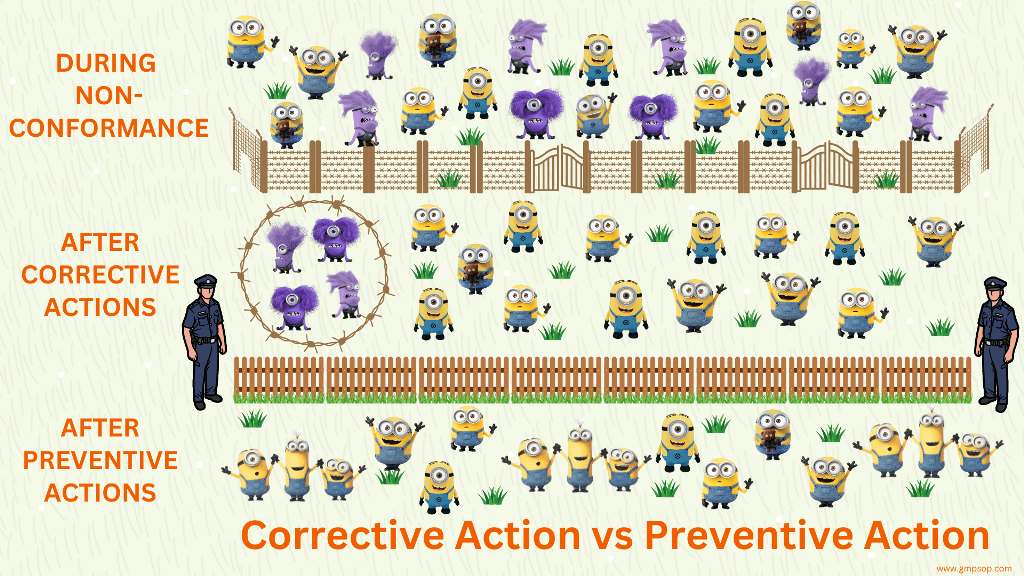
What is corrective and preventive action?
ISO 9000:2005 Sec. 3.6 defines them a follows:
– A correction is an action to eliminate a detected nonconformity.
– A corrective action is an action to eliminate the cause of a detected nonconformity, and
– A preventive action is an action to eliminate the cause of a potential nonconformity.
By definition, corrective actions are required to eliminate the causes of an existing nonconformity, defect, or other undesirable situation. Corrective actions are taken to fix a defect on hand.
On the other hand, preventive actions are implemented to prevent potential non-conformances in the future. Preventive action is taken to fix the cause of a problem before it can happen. The future risk mitigation measures are clear examples of preventive action.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Corrective action vs preventive action examples
In pharmaceuticals, corrective and preventive action (CAPA), is conducted at the implementation stage of most quality concern investigations.
Corrective and preventive actions are identified and implemented as the outcome of investigations such as deviations, product complaints, internal audit observations, risk assessments, stability failure, or other process or system-related non-conformances.
Let’s review another example to understand the difference between correction, corrective and preventive actions.
In a packaging line, a printer was used to imprint manufacturing and expiry dates on a batch of empty cartons which will be used to fill tablet strips.
During the printing operation, QA Inspector found the expiry dates on some cartons were illegible and the ink was smudged.
The problem was notified to Supervisor. As an immediate correction, the printing operation was halted. The printing line was separated from other nearby lines.
A 100% inspection has revealed that 25% of the printed cartons have the same defect.
As part of corrective actions, the defective cartons were quarantined. A quick reconciliation was carried out in the following way,
Number of issued cartons = number of unused cartons on the line + number of defective cartons.
The good cartons were also quarantined and returned to the warehouse in case they could be used in the future.
After the root cause investigation, it was identified the printer was doing its job correctly. However, the specified printing area on the carton was too glossy for the printer to make a clear impression and the ink was smudging.
Further to corrective action, the defective packaging (carton) lot was destroyed which was initially quarantined.
The entire unused packaging lot which was quarantined was returned to the supplier.
A fresh lot of unprinted cartons was issued for printing. QA Inspector found no defect on those.
As a preventive action, the supplier of the carton was contacted and presented the issue. The supplier has taken action in their manufacturing process to improve the printing area which should stop the recurrence of the printing defect in the future.
What are some corrective action examples in pharmaceuticals?
There is no limit to corrective actions in pharmaceuticals. They are depended on the issue or incidents. The primary objective of taking corrective actions is to eradicate the defect on hand.
Corrective actions are often required to improve factors such as man, machine, material, method, measurement, and environment of the pharmaceutical operation.
The following are some handpicked ones for the sake of example.
– Manufacturing: immediate corrective action on manufacturing process as a result of a deviation from a validated procedure or an out-of-specification result.
– Change control: implementing changes to equipment or procedures if deficiency is identified during investigation, such as updating a standard operating procedure or modifying a piece of equipment.
– Training: conducting training for personnel to ensure they understand the procedures and can perform their tasks correctly.
– Testing: performing additional testing or analysis to make a sound decision such as accepting or rejecting a batch.
– Process control: conducting process analytical research to identify potential areas for improvement and implementing changes as needed.
– SOP: reviewing and updating standard operating procedures to address potential deficiencies or gaps from regulatory expectations.
– Warehouse: resolving any discrepancies in inventory records, such as a discrepancy between the physical count and the recorded count.
– Status control: implementing status labels to clearly identify workflow status such as issues, returns, rejects and reworks of components to prevent mix ups.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
What are some preventive action examples in pharmaceutical?
Similar to corrective actions, preventive actions are taken where necessary for issues from which corrective actions have derived.
Following are some handpicked examples of preventive actions in pharmaceuticals.
– SOP: regularly review and update standard operating procedures to ensure they are accurate, up-to-date, and reflect best practices.
– Maintenance: conduct regular equipment maintenance and calibration to prevent breakdowns and ensure equipment is operating as intended.
– Training: prove ongoing training and development for personnel involved in the manufacturing process to ensure they have the knowledge and skills needed to perform their tasks correctly.
– Risk assessment: conduct risk assessments to identify potential hazards or areas of concern in the manufacturing process and develop plans to mitigate those risks.
– Manufacturing: implement quality assurance and control measures at various stages of the manufacturing process to identify and address any issues early on, before they become bigger problems.
– Process monitoring: regular monitoring and analyzing data related to the manufacturing process, such as production rates, quality metrics, and environmental factors, to identify trends or potential issues and take action as needed.
– Market action: conduct mock recalls and other contingency planning activities to ensure the organization is prepared to respond effectively in the event of a product recall or other crisis.
What is corrective and preventive action plan?
Pharmaceutical sites should establish and maintain standard operating procedures for corrective and preventive action activities to assure all incidents of nonconformity are identified, reported and investigated.
Root cause analysis tool is used for non-conformance investigation which leads to the identification of corrective actions.
The effectiveness of the implemented corrective actions should be evaluated periodically to ensure the same incident would not occur in the future.
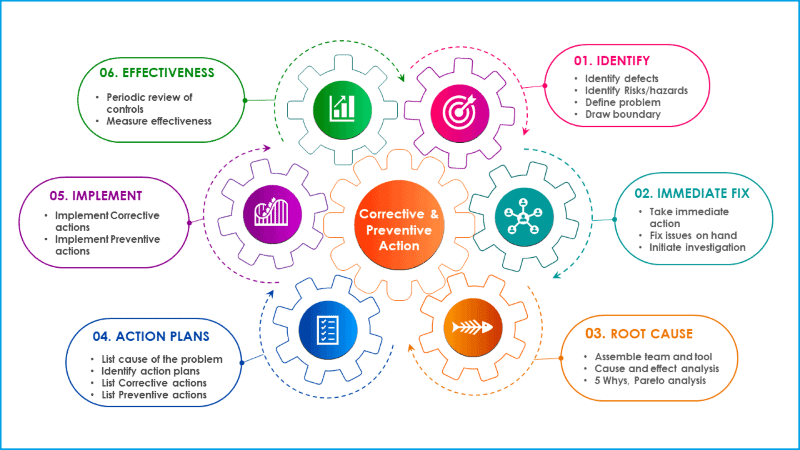
In general, corrective and preventive action process follows sequential steps starting from the identification of the problem, root cause analysis, identify mitigation actions and follow up the effectiveness of CAPA.
The corrective and preventive process includes the following sections in chronological order.
Step 1: Define the problem
This is the first section of corrective and preventive action process. At this, you should be asking a series of questions in order to define the problem, and its history and to outline any work that has been done so far.
Typical questions during the defining stage are:
– What is the problem / incident? (record exactly what actually happened)
– When did it happen? (record the date and time of incident)
– Where did it happened? (to record specifically what process, equipment or facilities were impacted)
– Who was working when the incident was discovered? (record who had found the incident first time)
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Step 2: Implement immediate fixes
In this step you should measure the gravity of the problem. You should determine the scope and boundary of the problem.
An understanding of immediate actions is prepared.
The following questions are helpful to understand what immediate steps are taken and why.
– What actions are taken immediately to fix the problem? (to record briefly the actions taken and by whom?)
– How long did it take to fix the problems? (to determine incident lost time)
– Has this happened before? (to identify history or trend on similar incidents)
– What actions were taken before? (to record any changes made previously)
– Which area of the processing line has been impacted?
– Are immediate actions sufficient to fix the issue?
Step 3: Conduct root cause analysis
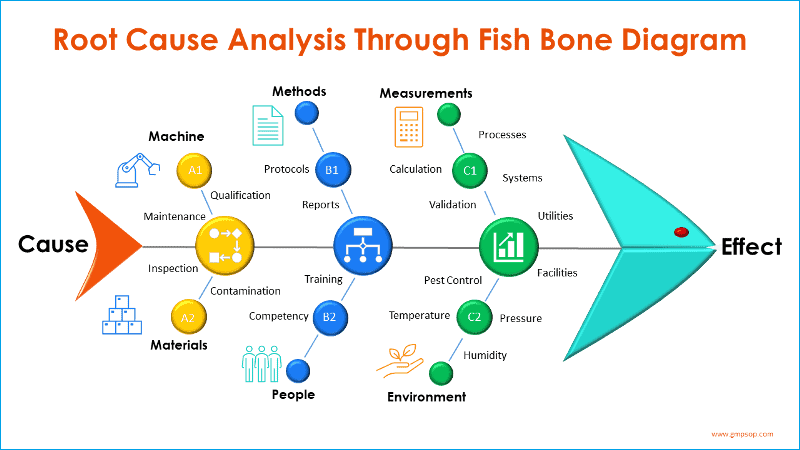
At this step, you as part of the investigation team should carry root cause analysis. Typically called cause and effect analysis (Fishbone diagram, also known as Ishikawa diagrams).
The process starts by adding the effect (defined problem) at the front of the fish head and creating branches of possible causes from the six process areas and systems.
Participants brainstorm on each of six broad categories or systems which are:
– Machine
– Methods
– Measurements
– Materials
– People, and
– Environments
A fishbone diagram is a visual tool and the exercise is particularly helpful in identifying possible categories of causes of a problem that might otherwise be not too obvious.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Step 4: Prepare an action plan
When you will reach to the potential solutions group them in order of difficulty to implement. Not every solution would have equal impact on eradicating the root cause. Some are easy to implement and potential improvement is significant. Some are harder to implement and the possible return on investment would be less significant.
You and the investigation team should come up with a list of action plans (corrective and preventive actions) and justify which would have the most practical impact on removing the defects.
Step 5: Implement an action plan
Once the corrective and preventive action plans are prepared, you should implement the action plans. For instance, you can undertake a list of process improvement works targeting the elimination of causes that have resulted in the defect.
Some preventive actions which are beneficial over the long term can also be implemented at the same time to prevent a potentially similar type of other defects.
Step 6: Follow up action plan
The final stage of corrective and preventive action process is to periodically follow up the effectiveness of the actions implemented earlier. You should prepare a schedule to revisit the corrective and preventions and check if the plans were not deviated from the target. Also you should measure if the root causes have been eliminated completely from the process.
What is corrective and preventive action procedure?
The corrective and preventive action procedures outlines a structured approach to identify and resolve quality and safety issues that may arise in the manufacturing process.
CAPA procedure goes above and beyond merely describing a CAPA plan. It is a complete management of corrective and preventive action process.
The CAPA procedure typically include the following key steps:
– Responsibilities for managing CAPA life cycle
– When to raise CAPA?
– How to complete a CAPA decision tree?
– Maintaining CAPA register
– CAPA plan which includes,
> Identification of the problem
> Investigational approach
> Corrective action plan
> Implementation strategy
> Preventive action (where necessary)
> CAPA effectiveness monitoring
– How to conduct root cause investigation?
– Maintaining CAPA documentation e.g. CAPA report
– CAPA effectiveness checks
– CAPA closure and ongoing review
What should be included in your CAPA procedure?
1. CAPA responsibilities
Top Management shall make a decision as to whether to take action and what action to take, considering the magnitude of problems and likely risk and initiating corrective and preventative actions (CAPA) where applicable.
The Regulatory Department is responsible for recording of changes to documented procedures resulting from corrective and preventative action.
Top Management shall determine the corrective or preventative action needed to address or prevent a particular or potential non-conformity, including review of data and data trend on non-conforming product.
The Regulatory Department shall ensure that all relevant information on actions taken is submitted for management review.
The Management Representative shall ensure effective operation of CAPA as part of Management review.
2. When to raise CAPA?
When material, component, product, pack, documentation or procedure is found to be unsuitable for the required purpose or process, those need to be reviewed and improved through CAPA procedure.
A CAPA shall be raised to determine the potential root cause and the corrective and/or preventive action when the CAPA may be related to a system or process failure where:
– It is considered that the corrective or preventative action cannot be completed within 60 days of identification of the root cause, using day-to-day resource allocation within the owning department
– The corrective or preventative action will require the establishment of a cross-functional or inter-site team
– The non-conformance impacts on regulatory compliance
– There is a significant chance of the non-conformance recurring
– The defect is likely to lead to significant customer dissatisfaction
– The corrective or preventative action requires a request to be raised for unbudgeted capital expenditure
– An internal or external regulatory audit, inspection or supplier/customer audit which results in non-conformances or observations requiring corrective and preventative actions
A CAPA shall be raised as a result of trends in data from the following sub-systems:
– Non-conformities (including internal, external and supplier audits)
– Customer complaints
– Service and repair reports
– Post-market surveillance
– Deviation authorizations
– Customer returned product
– Management review
– Process non-conformances
– Quality system non-conformances
– Overviews of important and common problems from group audits
3. CAPA project management
The nature and extent of the investigation and actions taken shall be proportionate to the significance of the problem. The significance may be determined through a documented risk review. The CAPA shall be assigned to the respective manager.
4. CAPA decision tree
You should add a section in the procedure called CAPA decision tree.
This will instruct employees to complete a decision tree by circling decision points regarding the source of non-conformity, the obviousness of probable root cause, whether the issue is a systematic issue and an assessment of risk.
If a CAPA file is to be raised, the signed CAPA decision tree is sent to the regulatory department.
5. CAPA file initiation
A CAPA number shall be issued in the format of CAPA/YY/nnn (where nnn is a sequential number held in the CAPA master file, and YY is the last two digits of the calendar year) and documented on in the CAPA Log when presented with information that meets the requirements for CAPA initiation by the regulatory department.
A CAPA Project Checklist shall be assigned for each file.
A corrective and preventive action form shall be sent to the owner to complete section one (including entering the CAPA number assigned and any relevant information including the source of the CAPA (ie. Non-conformance number, complaint number, audit information and any preliminary investigation findings).
6. Root Cause Investigation
The CAPA owner shall be responsible for performing the root cause investigation.
The following guidelines shall be used during completion of section two:
– Detail a description of the event and date
– List similar events noted (if applicable)
– Explain how the event may impact other areas, products or sites
– List probable causes
If the root cause is not obvious, a root-cause analysis should be performed.
The CAPA owner shall indicate in section two of the CAPA Form which of the root cause analysis tools have been used and what the potential root causes are.
Once complete, the form shall be sent to the regulatory department to obtain approval(s).
All actions taken shall be documented in the CAPA file indicating whether these actions are Investigative, Corrective, or Preventative.
7. CAPA action plan and approvals
Determine if corrective or preventative action should be taken.
If no additional action is required, a justification shall be documented and the required approvals should be obtained.
If actions are to be taken, detail those action plan on the CAPA form.
The action plan(s) require approval prior to implementation.
Obtain the required approvals listed in section four of the CAPA Form.
8. Documentation
All corrective and preventive actions to be taken as part of a CAPA process must be completed in accordance with applicable procedures in the business area and documented in the CAPA form.
9. CAPA effectiveness check
After implementation of the corrective or preventive action, the effectiveness should be verified using the appropriate means, e.g.
– From the source of the non-conformity, compare the data before and after the implementation of the preventative action(s).
– Process or documentation audit
Give a sufficient amount of time (typically one month) in order to have an acceptable amount of data to make a judgment.
If there has been any recurrence of the identified problem after the corrective and/or preventative actions have been established and implemented, the root cause and actions must be reviewed and the CAPA should not be considered ready for closure.
If the effectiveness check is successful, proceed to CAPA closure.
If the effectiveness check is unsuccessful, the CAPA shall not be closed. The CAPA will be forwarded to the owner for further investigation.
10. CAPA Closure
Upon successful effectiveness check, obtain the required CAPA closure signatures.
The CAPA file can be considered for closure when the following information is available:
– The CAPA project checklist has been completed and signed off
– The CAPA decision tree has been completed and signed off
– The CAPA form has been completed, the effectiveness check(s) were successful and all required signatures are received.
11. CAPA review
The CAPA review shall take place in accordance with the management review meetings.
The following information shall be reviewed at the meeting:
– All new CAPA proposals shall be submitted for review. These CAPAs shall be ratified or rejected based on the significance of a related event or emerging trend (risk). If necessary, for newly accepted CAPAs, action owners and resources should be assigned.
– A documented justification for rejecting a CAPA proposal should be documented.
– Resolution of issues that are adversely affecting CAPA action completion.
– Effectiveness reviews and closure of CAPAs.
You should escalate the cases to top management where corrective actions are past their respective due dates. So that top management is aware of the implications for process quality and the resources required to address the slippage.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Conclusion
Corrective and preventive action (CAPA) is a critical component of quality management system in the pharmaceutical industry.
CAPA procedure in the pharmaceutical industry is mandated by regulatory agencies. You have to have one and use the procedure for continuous improvement. Failure to comply with regulatory requirements can result in penalties, fines, and damage to the company’s reputation.
CAPA actions help to ensure that any quality or safety issues are identified, analyzed, resolved, and prevented from reoccurring.
A CAPA plan must be embedded into a CAPA procedure. The key steps of a CAPA plan are:
– Identify and define the problem
– Apply immediate fixes
– Conduct a root cause analysis
– Prepare a corrective and preventive action plan
– Implement those action plan
– Follow up on the effectiveness of those actions.
By definition, corrective actions are required to eliminate the causes of an existing nonconformity, defect, or undesirable situation. Corrective actions are taken to fix a defect on hand.
On the other hand, preventive actions are implemented to prevent potential non-conformances in the future. Preventive action is taken to fix the cause of a problem before it can happen. The future risk mitigation measures are clear examples of preventive action.
In pharmaceutical industry, corrective and preventive action (CAPA), is conducted at the implementation stage of most quality concern investigations.
In most cases, corrective and preventive actions are identified and implemented as the outcome of investigations such as deviations, product complaints, change management, audit observations, risk assessments, stability failure, or other process or system-related non-conformances.
In addition to the actual CAPA plan, your CAPA procedure should address the area, such as:
– Who should be responsible for raising, investigating, approving, and implementing CAPA?
– When to raise CAPA?
– How to complete a CAPA decision tree?
– Maintaining the CAPA register
– How to conduct a root cause investigation?
– Maintaining CAPA documentation e.g. CAPA report
– CAPA effectiveness checks
– CAPA closure and ongoing review
Corrective and preventive action procedure is essential to the success of pharmaceutical manufacturing operations.
They help identify and address quality and safety issues, preventing them from reoccurring in the future. A robust CAPA process is an ongoing effort, with a focus on continuous improvement, and is a key requirement for compliance with regulatory bodies.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.

Very comprehensive article on CAPA