
Data entry on quality control laboratory records
- Kazi
- Last modified: March 14, 2025
Table of Contents
Rules for completing laboratory records
According to GMP rules, laboratory records must be completed fully and dated appropriately to allow for a proper audit trail of events.
If a product or batch fails, the company may investigate by examining the laboratory records. Incomplete records may hinder such investigations.
On all laboratory records, personnel must include:
– Description (lot number and standard name (and identity of sample source.
– Statement of the test method.
– Weights and measures for preparation of samples, standards, and reagents.
– Graphs, printouts, and chromatograms annotated by the lot number, date, and signature of the analyst.
– All calculations, including signed printouts of validated spreadsheets.
– Initials or signature of the person performing each test and the date each test was performed.
Notebooks and worksheets are essential for supporting and verifying raw data and results’ accuracy, reliability, and authenticity.
Laboratory records are important for the following reasons:
– Providing documented evidence that tests were conducted.
– Allowing checks by a second analyst to be undertaken.
– Showing the traceability of standards and samples.
– Allowing the reliable investigation of OOS events.
– Troubleshooting problems and investigating complaints.
These records can also be used as legal evidence of compliance.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Verification of calculations in laboratory records
Because laboratory information accuracy is crucial, a second person (usually a senior analyst or supervisor) must review the laboratory record for completeness and compliance with established standards.
Specifically, the following must be checked during a review:
– Test method conditions must match the approved method (e.g., mobile phase, wavelength, column).
– Calculations are accurate.
– Results are compliant with the approved specifications.
– No data or results have been omitted without full review and approval.
– System suitability checks are within documented limits.
– Assays done in duplicate are within the internal limits for allowed differences between the assays.
– Deviations to the test method were properly recorded and did not affect the final results.
– If measurements have been changed, the reasons are properly documented and authorized.
Altering rules for laboratory records
To alter a paper-based laboratory record, personnel must abide by the following rules:
– Any change made to a record must be signed and dated.
– Any change made to a record must permit reading of the original information.
– The reasons for the change must also be recorded.
– Cross out changes with a single line, date, and initial the change.
– Do not write over the original information.
– Ensure that all changes are legible.
Any changes to electronic records should be made according to a defined procedure.
The procedure should include a provision for validating, checking, approving, and implementing the change.
The accuracy of the changes should still be checked either by a second operator or supervisor or by validated electronic means.
i. Significant figures and rounding
Regulated laboratories have rules for how to handle significant figures and tolerances:
– Always refer back to the limits or specifications when rounding results. (Note: Limits are fixed numbers that are never rounded off).
– Report the result to the same number of decimal places in the specification.
– Complete all calculations before rounding off.
ii. Rounding calculations
– Rounding up: If the last digit equals or exceeds 5, eliminate it and index up the previous digit.
– Rounding down: If the last digit is smaller than 5, eliminate it and report to the unchanged previous digit.
The table below shows an example of the practice rounding rules.
Given a specification of >= 98.0%:
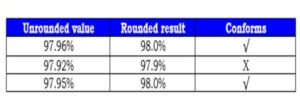
iii. Significant figures and tolerance
Analytical results observed in the laboratory (or calculated from experimental measurements) are compared with stated limits to determine whether there is conformance with compendia assay or test requirements.
The observed or calculated values usually will contain more significant figures than there are in the stated limit, and an observed or calculated result is to be rounded off to the number of places that are in agreement with the limit expression by the following procedure. (NOTE – Limits, fixed numbers, are not rounded off).
When rounding off is required, consider only one digit in the decimal place to the right of the last place in the limit expression.
If this digit is smaller than 5, it is eliminated, and the preceding digit is unchanged. If this digit is greater than 5, it is eliminated, and the preceding digit is increased by one. If this digit equals 5, the 5 is eliminated, and the preceding digit is increased by one.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Archiving rules for laboratory records
Laboratory records must be archived for at least the legal period.
The minimum period for archiving testing records is one year past the expiration date (or to your corporate requirements) of the tested batch or material.
This includes all supporting raw data and graphs, chromatograms, etc.
Other documents that must also be archived include:
– Test methods
– Specifications
– Stability records
– Validation protocols and reports
– Calibration, standardization, and training records
Examples of regulatory citations regarding laboratory data
When regulators audit quality control laboratories, they are particularly interested in testing data’s integrity, availability, accuracy, and traceability.
It is critical for any laboratory to properly manage, cross-check, maintain, and archive records and data.
Regulatory citations on laboratory data
i. Missing data:
– The original chromatographic data was not recorded in lab books and, therefore, unavailable for inspections.
– The SOP does not specify worksheet identification of each lab instrument used in the analysis.
– There is no record of the identity of equipment used to perform finished product testing.
– Records were not maintained for lot numbers and the pharmacopoeial status of standards used for product testing or equipment calibration.
– Certain lab reports were omitted from analyst notebooks.
– Raw data for HPLC assays was deleted from the computer system, and backup tapes were not maintained.
– HPLC chromatogram, computer report, and analytical procedure do not contain identifying numbers, allowing for reference to laboratory notebooks
ii. Data compliance:
– The stability data notebooks were not signed by reviewers.
– Laboratory worksheets do not allow a record of the method performed and calculations used.
– There is no provision for maintaining hard copy printouts of absorbance values used in calculating finished product assay results.
– Failure to identify different equipment, test methods, and testing facilities from those submitted in the application
– The content uniformity computer report’s dates differed from the laboratory notebook.
Laboratory e-records
Individual passwords should never be accessible to other analysts in the laboratory.
Paper-based laboratory records should state the names of the analysts responsible for the tests and/or the record’s completion. E-records are no different.
If someone logs on to a computer with someone else’s login, it is tantamount to forging a written record with another analyst’s signature.
Most laboratories rely heavily on automated systems to control instruments, capture raw data, calculate assays, release results, and archive information.
Regulatory agencies are interested in how these electronic systems are controlled and have specific rules governing computer system electronic records and electronic signatures.
These rules are often called the ”Port 11 Rules” after the FDA regulation, CFR Port 11.
The laboratory also has specific password protection rules for the use and security of records:
– Personnel should change their passwords regularly (e.g. every 90 days).
– Personnel should not display or share passwords.
– Upon leaving, personnel should log out of computer workstations.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Raw data Vs Derived data
Raw data on laboratory records may include photographs, microfilm or microfiche copies, computer printouts, magnetic media including dictated observations, and recorded data from automated instruments.
Raw data can include:
– Original entries into analyst workbooks, weighing logs, and calculations
– Original data recorded and stored by data loggers or other computer/electronic devices
– Instrument calibration, service, maintenance, and repair records
– Original recordings of such parameters as animal body weights
– Handwritten transcriptions to paper records displayed as a digital readout on equipment
– HPLC or other instrumental t racings or charts and integrator outputs from instruments
– Recorded visual observations
– Photographs (where used as a recording device)
Derived data is interpolated, extrapolated, or summarized from raw data.
Calculation summaries on a spreadsheet are an example of derived data.
Laboratory records in computerized systems
The use of computerized laboratory data acquisition systems is not new and is addressed in the following documents:
– EU/PICs Code of GMP – Annex 11
– GAMP (Good Automated Manufacturing Practices) Guide for Validation of Automated Systems
– US EPA 2185 -Good Automated Laboratory Practices
– US FDA Compliance Policy Guides for Computerized Drug Processing:
– 7132a.07 Input / Output checking
– 7132a.08 identification of “Persons” on batch Production and Control Records
– 7132a.11 CGMP Applicability to Hardware and Software
– 7132a. 12 Vendor Responsibility
– 7132a.15 Source Code for Process Control Application Programs
– FDA Guide to Inspection of Computerized Systems in Drug Processing
i. ERES rules
The CFR Part 11 subsections listed below cover the fundamental ERES rules that laboratory personnel should be aware of:
– Completeness of electronic records
– Availability of records
– Validation procedures
– Available in both human-readable and electronic form
– Ensuring accuracy/availability through the retention period
– Limits on system access
– Independent recording of transactions
– Sequence checks
– Adequate authority checks
– Electronic signature types supported
ii. Instrument that records raw data
Part 11 rules apply to almost all raw and derived laboratory data stored or manipulated electronically.
Part 11 rules would apply to the following:
– Digital readouts stored in e-files
– HPLC or other instrumental charts and integrator outputs that are stored as e-files
– Calculation spreadsheets
– Laboratory information management systems (LIMS)
– Environmental data loggers
iii. HPLC-generated electronic information
– Reference to the sample numbers
– Test conditions (e.g., column, strength, wavelength)
– Sample run order
– Summary table of results
– Assay calculations and results
– A chromatogram showing peaks and integration
iv. Storing raw data
The quality control rules for storing raw data include:
– Retain all raw data for defined periods.
– Structure the archives for orderly storage, indexing, and expedient retrieval.
– Ensure that the storage conditions protect data from loss or deterioration
– Restrict access to authorized personnel
– Identify an individual to be responsible for maintaining the archives.
v. Requirements for storing electronic data
There are also quality controls for storing electronic data drawing on the Part 11 rules, which include:
– Protect data from unapproved changes or corruption.
– Ensure that any changes are audit-trailed and password-controlled.
– Ensure that all records are retrievable, even during a software upgrade.
– Restrict access to authorized personnel.
– Ensure that there is a documented regular backup strategy.
– Ensure that the data can be retrieved in readable form.
vi. Security and authenticity of laboratory records
Some basic guidance on security and authenticity issues for computerized systems:
– Only authorized individuals can make data entries.
– Data entries may not deleted. Changes must be made in the form of amendments.
– The database must be made as tamperproof as possible.
– The SOPs must describe the procedures for ensuring the validity of the data.
Validation of laboratory computerized data acquisition requires comparing data from the specific instrument with the same data electronically transmitted through the system and emanating on a printer.
Periodic data comparison would be sufficient only when it has been made over a sufficient period of time to ensure that the system produces consistent and valid results.
(source: FDA Inspection Guide: Pharmaceutical Quality Control Labs (7/93))

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
