Embed Codes - gmpsop
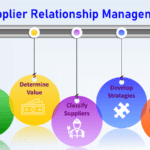
Supplier Relationship Management.
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-develop-supplier-relationship-management-strategies-in-gmp/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Supplier Relationship Management" width="800px" border="0" title="Embed Code - GMPSOP 2" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/03/Supplier-Relationship-Plans.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/03/Supplier-Relationship-Plans.png" alt="Supplier Relationship Management" width="800px" border="0" title="Embed Code - GMPSOP 2"></noscript></a></p>
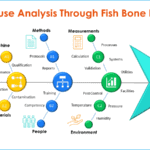
Fishbone diagram for root cause analysis
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-conduct-a-root-cause-investigation/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Fishbone diagram for investigation" width="800px" border="0" title="Embed Code - GMPSOP 4" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/03/Fishbone-diagram-for-investigation.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/03/Fishbone-diagram-for-investigation.png" alt="Fishbone diagram for investigation" width="800px" border="0" title="Embed Code - GMPSOP 4"></noscript></a></p>
Supplier Relationship Infographics
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-develop-supplier-relationship-management-strategies-in-gmp/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Supplier Relationship Management" width="800px" border="0" title="Embed Code - GMPSOP 6" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/03/Supplier-Relationship-Infographic.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/03/Supplier-Relationship-Infographic.png" alt="Supplier Relationship Management" width="800px" border="0" title="Embed Code - GMPSOP 6"></noscript></a></p>
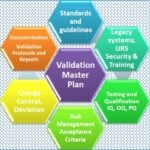
Master validation plan
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/creating-a-master-validation-plan/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20672%200'%3E%3C/svg%3E" alt="Master Validation Plan" width="672px" border="0" title="Embed Code - GMPSOP 8" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2021/02/Master-Validation-Plan.jpg"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2021/02/Master-Validation-Plan.jpg" alt="Master Validation Plan" width="672px" border="0" title="Embed Code - GMPSOP 8"></noscript></a></p>console.log( 'Code is Poetry' );
Understanding Quality Assurance
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/basic-understanding-of-quality-management/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20960%200'%3E%3C/svg%3E" alt="Quality Assurance Understanding" width="960px" border="0" title="Embed Code - GMPSOP 10" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/04/Quality-Assurance-Understanding.jpg"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/04/Quality-Assurance-Understanding.jpg" alt="Quality Assurance Understanding" width="960px" border="0" title="Embed Code - GMPSOP 10"></noscript></a></p>
Cross Contamination in GMP
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/cross-contamination-prevention-guidelines/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20605%200'%3E%3C/svg%3E" alt="Cross Contamination in GMP" width="605px" border="0" title="Embed Code - GMPSOP 12" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/03/Cross-Contamination-in-GMP.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/03/Cross-Contamination-in-GMP.png" alt="Cross Contamination in GMP" width="605px" border="0" title="Embed Code - GMPSOP 12"></noscript></a></p>
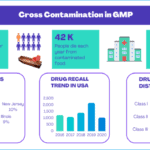
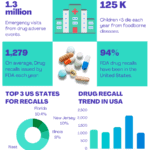
Cross Contmination data
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/cross-contamination-prevention-guidelines/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20900%200'%3E%3C/svg%3E" alt="Cross Contmination data" width="900px" border="0" title="Embed Code - GMPSOP 14" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2021/11/Cross-Contmination-data-2.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2021/11/Cross-Contmination-data-2.png" alt="Cross Contmination data" width="900px" border="0" title="Embed Code - GMPSOP 14"></noscript></a></p>
Cross Contamination controls
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/cross-contamination-prevention-guidelines/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20900%200'%3E%3C/svg%3E" alt="Cross Contamination controls" width="900px" border="0" title="Embed Code - GMPSOP 16" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2021/11/Cross-Contamination-controls-1.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2021/11/Cross-Contamination-controls-1.png" alt="Cross Contamination controls" width="900px" border="0" title="Embed Code - GMPSOP 16"></noscript></a></p>
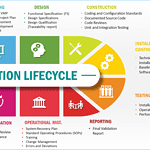
Master Validation Plan
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/creating-a-master-validation-plan/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20797%200'%3E%3C/svg%3E" alt="Master Validation Plan Infograph" width="797px" border="0" title="Embed Code - GMPSOP 18" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2024/06/Master-Validation-Plan.jpg"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2024/06/Master-Validation-Plan.jpg" alt="Master Validation Plan Infograph" width="797px" border="0" title="Embed Code - GMPSOP 18"></noscript></a></p>
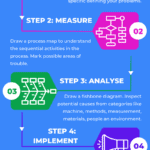
DMAIC Root Cause Investigation
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-conduct-a-root-cause-investigation/'><img width="800" height="2000" decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%202000'%3E%3C/svg%3E" alt="DMAIC Root Cause Investigation" border="0" title="Embed Code - GMPSOP 20" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/03/DMAIC-Root-Cause-Investigation.png"><noscript><img width="800" height="2000" decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/03/DMAIC-Root-Cause-Investigation.png" alt="DMAIC Root Cause Investigation" border="0" title="Embed Code - GMPSOP 20"></noscript></a></p>
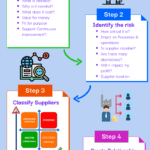
Supplier Selection Process
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/seven-steps-to-complete-a-supplier-selection-process-in-gmp/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Supplier Selection Process" width="800px" border="0" title="Embed Code - GMPSOP 22" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/04/Supplier-Selection-Process.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/04/Supplier-Selection-Process.png" alt="Supplier Selection Process" width="800px" border="0" title="Embed Code - GMPSOP 22"></noscript></a></p>
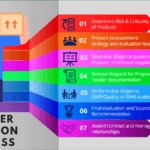
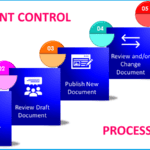
Document Control Process in GMP
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/document-control-process-in-gmp-environment/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Document Control Process in GMP" width="800px" border="0" title="Embed Code - GMPSOP 24" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/04/Document-Control-Process-in-GMP.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/04/Document-Control-Process-in-GMP.png" alt="Document Control Process in GMP" width="800px" border="0" title="Embed Code - GMPSOP 24"></noscript></a></p>
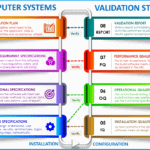
Computer System Validation
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-computer-system-validation-csv-in-gmp/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201280%200'%3E%3C/svg%3E" alt="What is Computer System Validation" width="1280px" border="0" title="Embed Code - GMPSOP 26" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2021/07/What-is-Computer-System-Validation.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2021/07/What-is-Computer-System-Validation.png" alt="What is Computer System Validation" width="1280px" border="0" title="Embed Code - GMPSOP 26"></noscript></a></p>
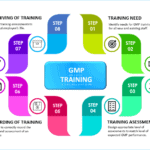
GMP Training Steps for employees
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-conduct-gmp-training-for-employee/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="GMP Training Steps for employee" width="1024px" title="Embed Code - GMPSOP 28" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/04/GMP-Training-Steps-for-employees.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/04/GMP-Training-Steps-for-employees.png" alt="GMP Training Steps for employee" width="1024px" title="Embed Code - GMPSOP 28"></noscript></a></p>
GMP Training for Employees
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-conduct-gmp-training-for-employee/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="GMP Training for employee" width="800px" title="Embed Code - GMPSOP 30" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/04/GMP-Training-for-Employees.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/04/GMP-Training-for-Employees.png" alt="GMP Training for employee" width="800px" title="Embed Code - GMPSOP 30"></noscript></a></p>
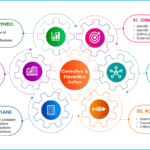
Corrective and preventive action process
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/six-step-procedure-for-corrective-and-preventive-action/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Corrective and preventive action process" width="800px" title="Embed Code - GMPSOP 32" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/05/Corrective-and-preventive-action-process.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/05/Corrective-and-preventive-action-process.png" alt="Corrective and preventive action process" width="800px" title="Embed Code - GMPSOP 32"></noscript></a></p>
Quality Risk Management in Validation
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-use-quality-risk-management-in-validation-testing/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Quality Risk Management in Validation " width="800px" title="Embed Code - GMPSOP 34" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/08/Quality-risk-management-in-validation.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/08/Quality-risk-management-in-validation.png" alt="Quality Risk Management in Validation " width="800px" title="Embed Code - GMPSOP 34"></noscript></a></p>
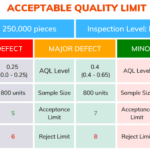
Acceptable Quality Limit (AQL)
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-acceptable-quality-limit-aql-in-samping/'><img width="800" height="450" decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%20450'%3E%3C/svg%3E" alt="Acceptable Quality Limit (AQL)" title="Embed Code - GMPSOP 36" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/09/Acceptable-Quality-Limit.png"><noscript><img width="800" height="450" decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/09/Acceptable-Quality-Limit.png" alt="Acceptable Quality Limit (AQL)" title="Embed Code - GMPSOP 36"></noscript></a></p>
Acceptable Quality Limit Sampling plan
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-acceptable-quality-limit-aql-in-samping/'><img width="800" height="488" decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%20488'%3E%3C/svg%3E" alt="Acceptable Quality Limit Sampling plan" title="Embed Code - GMPSOP 38" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/09/Acceptable-Quality-Limit-Sampling-plan.png"><noscript><img width="800" height="488" decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/09/Acceptable-Quality-Limit-Sampling-plan.png" alt="Acceptable Quality Limit Sampling plan" title="Embed Code - GMPSOP 38"></noscript></a></p>
Sample Size Code Letter
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-acceptable-quality-limit-aql-in-samping/'><img width="572" height="343" decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20572%20343'%3E%3C/svg%3E" alt="Sample Size Code Letter" title="Embed Code - GMPSOP 40" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/09/Sample-Size-Code-Letter.jpg"><noscript><img width="572" height="343" decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/09/Sample-Size-Code-Letter.jpg" alt="Sample Size Code Letter" title="Embed Code - GMPSOP 40"></noscript></a></p>
Single Sampling Plan for Normal Inspection
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-acceptable-quality-limit-aql-in-samping/'><img width="572" height="409" decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20572%20409'%3E%3C/svg%3E" alt="Single Sampling Plan for Normal Inspection" title="Embed Code - GMPSOP 42" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/09/Single-Sampling-Plan-for-Normal-Inspection.jpg"><noscript><img width="572" height="409" decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/09/Single-Sampling-Plan-for-Normal-Inspection.jpg" alt="Single Sampling Plan for Normal Inspection" title="Embed Code - GMPSOP 42"></noscript></a></p>
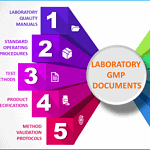
Laboratory Documentation Hierarchy
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-develop-quality-control-method-and-specifications-for-a-laboratory/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Laboratory Documentation Hierarchy" width="800px" title="Embed Code - GMPSOP 44" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2020/12/Laboratory-Documentation-Hierarchy.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2020/12/Laboratory-Documentation-Hierarchy.png" alt="Laboratory Documentation Hierarchy" width="800px" title="Embed Code - GMPSOP 44"></noscript></a></p>
Reference Standards
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-meant-by-reference-standard-in-pharmaceuticals/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Reference Standard" width="800px" title="Embed Code - GMPSOP 46" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/11/Reference-Standard.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/11/Reference-Standard.png" alt="Reference Standard" width="800px" title="Embed Code - GMPSOP 46"></noscript></a></p>
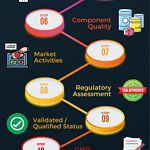
The ten steps of annual product quality review
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-prepare-sop-for-annual-product-quality-review-in-gmp/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="The ten steps of annual product quality review" width="800px" title="Embed Code - GMPSOP 48" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2020/12/The-ten-step-of-annual-product-quality-review.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2020/12/The-ten-step-of-annual-product-quality-review.png" alt="The ten steps of annual product quality review" width="800px" title="Embed Code - GMPSOP 48"></noscript></a></p>
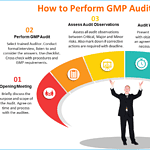
GMP Audit in Six Steps
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-perform-gmp-audit-in-six-steps/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="GMP Audit in Six Steps" width="800px" title="Embed Code - GMPSOP 50" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2020/12/GMP-Audit-in-Six-Steps.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2020/12/GMP-Audit-in-Six-Steps.png" alt="GMP Audit in Six Steps" width="800px" title="Embed Code - GMPSOP 50"></noscript></a></p>
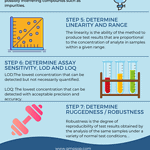
07 steps of analytical method validation
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-analytical-method-validation-in-gmp/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="07 steps of analytical method validation" width="800px" title="Embed Code - GMPSOP 52" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2020/12/07-steps-of-analytical-method-validation.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2020/12/07-steps-of-analytical-method-validation.png" alt="07 steps of analytical method validation" width="800px" title="Embed Code - GMPSOP 52"></noscript></a></p>
Sampling procedure in pharmaceuticals
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/sampling-procedure-for-non-sterile-products/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Sampling procedure in pharmaceuticals" width="800px" title="Embed Code - GMPSOP 54" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/01/Sampling-procedure-in-pharmaceuticals.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/01/Sampling-procedure-in-pharmaceuticals.png" alt="Sampling procedure in pharmaceuticals" width="800px" title="Embed Code - GMPSOP 54"></noscript></a></p>
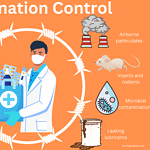
Contamination control in GMP
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/basic-overview-of-contamination-control-in-gmp-facility/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="Contamination control in GMP" width="1024px" title="Embed Code - GMPSOP 56" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/10/Contamination-control-in-GMP.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/10/Contamination-control-in-GMP.png" alt="Contamination control in GMP" width="1024px" title="Embed Code - GMPSOP 56"></noscript></a></p>
Concept of validation in pharmaceutical industry
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/concept-of-process-validation-for-pharmaceutical-industry/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201233%200'%3E%3C/svg%3E" alt="Concept of validation in pharmaceutical industry" width="1233px" title="Embed Code - GMPSOP 58" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/10/Validation-in-pharmaceutical-industry.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/10/Validation-in-pharmaceutical-industry.png" alt="Concept of validation in pharmaceutical industry" width="1233px" title="Embed Code - GMPSOP 58"></noscript></a></p>
Equipment qualification steps in pharmaceutical industry
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/concept-of-process-validation-for-pharmaceutical-industry/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201280%200'%3E%3C/svg%3E" alt="Equipment qualification steps in pharmaceutical industry" width="1280px" title="Embed Code - GMPSOP 60" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/10/Equipment-qualification-steps-in-pharmaceutical-industry.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/10/Equipment-qualification-steps-in-pharmaceutical-industry.png" alt="Equipment qualification steps in pharmaceutical industry" width="1280px" title="Embed Code - GMPSOP 60"></noscript></a></p>
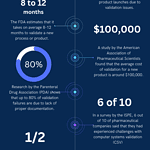
Statistical data on validation in pharmaceutical industry
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/concept-of-process-validation-for-pharmaceutical-industry/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Statistical data - validation in pharmaceutical industry" width="800px" title="Embed Code - GMPSOP 62" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/10/Statistical-data-validation-in-pharmaceutical-industry.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/10/Statistical-data-validation-in-pharmaceutical-industry.png" alt="Statistical data - validation in pharmaceutical industry" width="800px" title="Embed Code - GMPSOP 62"></noscript></a></p>
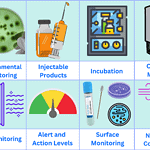
Environmental monitoring plan in GMP
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-environmental-monitoring-in-pharmaceutical-industry/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Environmental monitoring plan in GMP" width="800px" title="Embed Code - GMPSOP 64" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2022/12/Environmental-monitoring-plan.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2022/12/Environmental-monitoring-plan.png" alt="Environmental monitoring plan in GMP" width="800px" title="Embed Code - GMPSOP 64"></noscript></a></p>
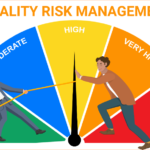
Quality Risk Management framework – ICH Q9
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-quality-risk-management-in-pharmaceutical/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20800%200'%3E%3C/svg%3E" alt="Quality Risk Management framework - ICH Q9" width="800px" title="Embed Code - GMPSOP 66" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2017/10/Quality-Risk-Management.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2017/10/Quality-Risk-Management.png" alt="Quality Risk Management framework - ICH Q9" width="800px" title="Embed Code - GMPSOP 66"></noscript></a></p>
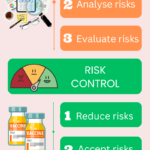
Quality risk management in pharmaceutical
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-quality-risk-management-in-pharmaceutical/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="Quality risk management in pharmaceutical" width="1024px" title="Embed Code - GMPSOP 68" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2017/10/Quality-risk-management-in-pharmaceutical.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2017/10/Quality-risk-management-in-pharmaceutical.png" alt="Quality risk management in pharmaceutical" width="1024px" title="Embed Code - GMPSOP 68"></noscript></a></p>
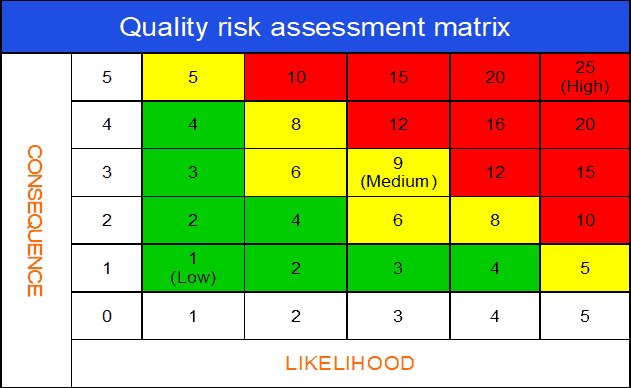
Quality risk assessment matrix
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/what-is-quality-risk-management-in-pharmaceutical/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20631%200'%3E%3C/svg%3E" alt="Quality risk assessment matrix" width="631px" title="Embed Code - GMPSOP 70" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2017/10/Quality-risk-assessment-matrix.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2017/10/Quality-risk-assessment-matrix.png" alt="Quality risk assessment matrix" width="631px" title="Embed Code - GMPSOP 70"></noscript></a></p>
GMP Warehouse Audit
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-prepare-for-a-gmp-warehouse-audit-tips-and-tricks/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="GMP Warehouse Audit" width="1024px" title="Embed Code - GMPSOP 72" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2023/04/GMP-Warehouse-Audit.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2023/04/GMP-Warehouse-Audit.png" alt="GMP Warehouse Audit" width="1024px" title="Embed Code - GMPSOP 72"></noscript></a></p>
GMP documentation in laboratory
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/typical-gmp-documentation-in-a-quality-control-laboratory/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="GMP documentation in laboratory" width="1024px" title="Embed Code - GMPSOP 74" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2020/12/GMP-documentation-in-laboratory.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2020/12/GMP-documentation-in-laboratory.png" alt="GMP documentation in laboratory" width="1024px" title="Embed Code - GMPSOP 74"></noscript></a></p>
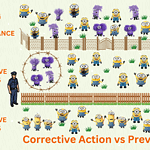
Corrective and preventive action
<p><strong>Please include attribution to https://www.gmpsop.com with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/corrective-and-preventative-action-procedure-for-good-manufacturing-practice/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="Corrective and preventive action" width="1024px" title="Embed Code - GMPSOP 76" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2020/12/Corrective-and-preventive-action.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2020/12/Corrective-and-preventive-action.png" alt="Corrective and preventive action" width="1024px" title="Embed Code - GMPSOP 76"></noscript></a></p>
Periodic review of validated processes
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/how-to-perform-periodic-review-of-systems-and-processes-in-pharmaceutical/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="Periodic review of validated processes" width="1024px" title="Embed Code - GMPSOP 78" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2021/11/Periodic-review-of-validated-processes.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2021/11/Periodic-review-of-validated-processes.png" alt="Periodic review of validated processes" width="1024px" title="Embed Code - GMPSOP 78"></noscript></a></p>
Equipment cleaning do’s and don’ts
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/some-tips-of-equipment-cleaning-in-pharmaceuticals-manufacturing-plant/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="Equipment cleaning do" width="1024px" s and title="Embed Code - GMPSOP 80" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2020/12/Equipment-cleaning-dos-and-donts.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2020/12/Equipment-cleaning-dos-and-donts.png" alt="Equipment cleaning do" width="1024px" s and title="Embed Code - GMPSOP 80"></noscript></a></p>
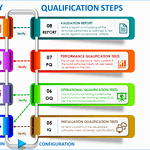
Installation qualification step by step
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/a-step-by-step-guide-to-successful-installation-qualification-iq/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%201024%200'%3E%3C/svg%3E" alt="Installation qualification step by step" width="1024px" title="Embed Code - GMPSOP 82" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2023/07/Installation-qualification-step-by-step.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2023/07/Installation-qualification-step-by-step.png" alt="Installation qualification step by step" width="1024px" title="Embed Code - GMPSOP 82"></noscript></a></p>
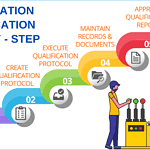
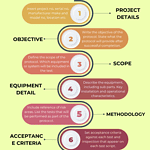
Installation and operational qualification protocol
<p><strong>Please include attribution to https://www.gmpsop.com/ with this graphic.</strong><br /><br /><a href='https://www.gmpsop.com/a-step-by-step-guide-to-successful-installation-qualification-iq/'><img decoding="async" src="data:image/svg+xml,%3Csvg%20xmlns='http://www.w3.org/2000/svg'%20viewBox='0%200%20880%200'%3E%3C/svg%3E" alt="Installation and operational qualification protocol" width="880px" title="Embed Code - GMPSOP 84" data-lazy-src="https://www.gmpsop.com/wp-content/uploads/2023/07/Installation-and-operational-qualification-protocol.png"><noscript><img decoding="async" src="https://www.gmpsop.com/wp-content/uploads/2023/07/Installation-and-operational-qualification-protocol.png" alt="Installation and operational qualification protocol" width="880px" title="Embed Code - GMPSOP 84"></noscript></a></p>