Overview of pharmaceutical quality control steps and implementations
- Kazi
- Last modified: March 13, 2025
Pharmaceutical quality control laboratories follow a series of procedural steps, beginning with the collection and storage of medicinal samples, validating test methods and testing equipment, qualifying the testing environment, training their analysts, documenting testing results against registered specifications, etc., prior to releasing medicinal batches to ensure they are safe, pure, effective, and traceable for human consumption.
Quality control in the pharmaceutical industry is the cornerstone of product safety and quality assurance. Hence, these functions are heavily regulated by government agencies. These quality control regulations are commonly known as good laboratory practice or GLP.
Quality control remains a vital part of quality assurance in pharmaceuticals, contributing to optimum performance in the production process. Sometimes, they are referenced interchangeably. Quality control and quality assurance form the foundation of total quality management.
From starting materials to testing pharmaceutical products coming out of the manufacturing process, quality control is the only function that detects unknown defects early and allows for timely correction.
It is the regulatory expectation that the quality control functions remain compliant with quality standards.
This article will focus on some of the common activities performed in quality control facilities daily to maintain the expected level of compliance and ensuring product quality. It will also elaborate on the steps you can take to make this function highly effective for product and patient safety.
Table of Contents
Key Takeaways
- The key objective of pharmaceutical quality control is to ensure that medicines are safe, pure, effective, and meet strict regulatory standards. Before they reach patients, every batch of products must meet the specified quality requirements. Therefore, the QC laboratory must follow good laboratory practice (GLP) guidelines.
- Personnel training and qualification are fundamental to quality control. Employees must be adequately trained for specific laboratory tasks and regularly requalified to ensure competency.
- Laboratory equipment must undergo thorough qualification and regular maintenance to ensure reliable operation. This includes design, installation, operational, performance checks, and routine recalibration before being used for routine testing. Regular performance checks will ensure that only qualified and calibrated equipment is used for testing.
- Test methods must be validated to ensure they consistently deliver accurate and reliable results. Method validation confirms that test procedures meet their intended purpose and comply with GLP standards. Validated test methods generate confidence in the results they are being used for testing.
- Proper management and handling of chemicals, reagents, volumetric solutions, and reference standards help maintain testing accuracy. These materials must be carefully sourced, labelled, stored, and disposed of according to strict procedures to prevent mix-ups, errors, or contamination.
- Comprehensive documentation is the backbone of pharmaceutical quality control. From specifications to test methods and standard operating procedures to test records and logbooks, the QC must maintain accurate and traceable documentation to ensure regulatory compliance
- Stability testing in the laboratory evaluates how the active ingredients in the product hold up over time under various storage conditions, such as temperature and humidity. These studies help determine expiry dates and ensure that medicines remain effective and safe throughout their shelf life, protecting patient safety.
- Laboratories must maintain clean and controlled environments to ensure the integrity of test results. This includes routine cleaning, environmental monitoring, and maintaining equipment and utilities according to GLP standards.
- All confirmed out-of-specification (OOS) results require immediate investigation to identify the root cause and determine corrective actions. The outcome of the investigation helps decide whether to resample, retest, or even use a new analyst.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Laboratory training
Laboratory management must ensure that each employee is educated, trained, and qualified to perform the functions assigned to them and remain competent to continue performing those functions.
– All employees must have training in GMP to a level relevant to their job function.
– Each position must have a generic training plan.
– Each employee must have an individual training plan.
– Training plans and competency assessment details (e.g., employee assay qualification) must be documented in an SOP for each assay the lab performs with pre-determined acceptance criteria.
– Qualification can occur via an operator validating an assay, receiving a tech transfer assay, having experience with a similar assay, or via parallel testing.
– Re-qualification requirements must be pre-defined (e.g., gown qualification).
– There must be a means of knowing if an employee is qualified to perform an assay.
– There must be a means of disqualifying employees from performing assays in which they were previously qualified if they cease demonstrating competency in the assay.
– There must be a mechanism to track and review operator errors. This review must be performed by Laboratory Management.
– Trainers must be qualified and approved to train.
Equipment qualification in pharma quality control
Critical or direct impact equipment in pharmaceuticals must be qualified. Automated equipment and computer systems should also be qualified before they are used in quality control data processing.
Laboratory equipment qualification is the collection of documented evidence that demonstrates that specific equipment performs suitably for its intended purpose.
Qualification is a logical step-wise process, which generally consists of the following stages:
– User Requirement Specifications (URS)
– Design Qualification
– Installation Qualification
– Operational Qualification
– Performance Qualification
The amount of documentation and level of detail may vary depending on the criticality of GMP and the complexity of the new or modified equipment.
Laboratory equipment must be qualified according to the validation master plan. Only qualified equipment can generate GMP test results.
Simple, “Complete Off The Shelf (COTS)” style equipment may be calibrated only (e.g., balance). Justification for this approach must be documented.
Instruments (e.g. thermometer) must be calibrated. The calibration frequency, limits, range of use and calibration points must be included in the site Master Instrument List.
Only calibrated instruments can be used to generate GMP test results.
The lab must have a process for ensuring the qualification and / or calibration status is maintained.
The movement of non-portable equipment within the lab must follow site change control procedures to address any requalification considerations.
Maintenance programs must be documented for laboratory equipment.
Test method validation in quality control
Test method validation generally means the same as analytical method validation, where the terms are interchangeable.
Test method validation in pharma quality control is the process by which it is established that the method’s performance characteristics, such as precision, accuracy, specificity, linearity, limit of detection (LOD), limit of quantitation (LOQ), and robustness, meet the requirements for the intended applications.
It is a regulatory requirement to produce evidence-based determination that the test methods you have employed to analyze your products are validated and free from quality issues. This means that the analytical methods consistently generate true results with precision and accuracy every time.
That is why regulatory bodies inspect test method validation programs to ensure laboratories operate under stringent GLP (Good Laboratory Practice) guidelines.
A planned analytical method validation process can generate high confidence in your products.
Basic validation philosophy
The basic validation philosophy states that establishing documented evidence provides a high degree of assurance that a specific process (in this case, analytical test method) will consistently produce a product (i.e., assay result) meeting its predetermined specifications and quality attributes (i.e., accuracy, precision, etc.)
FDA – Process Validation Guidelines (published in January 2011) are crucial for maintaining the quality management system.
Following are the pre-requisites for test method validation in pharmaceutical quality control:
– Your laboratory instruments are qualified
– You have a well-developed documentation process
– Your reference standards are reliable, stable
– You have trained and qualified analysts
– Be aware that the test method is robust to factors that may change or be uncontrolled
– You have a well-documented method validation protocol and reporting system
Classes of test methods requiring validation
You need to identify those test methods used to determine your product’s quality attributes and the efficacy of the processes that directly or indirectly impact the quality of your products. Then, include those test methods for the validation regime.
For example:
– Test methods for the identity of active pharmaceutical ingredients (API)
– Qualitative measurements for impurities/degradants
– Limit tests for impurities/degradants
– Quantitative assays of an API or drug product active material
– Physical product characteristics, e.g. dissolution rate
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Laboratory reference standards
A reference standard is a specific batch of a substance of known composition and purity to which responses of samples of unknown composition or purity are compared to determine the concentration or purity of that unknown composition.
Pharmaceutical quality control laboratories use two types of reference standards for their testing purpose.
– Primary reference standard.
– Secondary reference standard.
Reference standards must be traceable to official sources (e.g., USP, Phr. EU, etc.)
You should establish a standard operating procedure to describe the management of reference standards in the laboratory.
Management of reference standards should have the minimum information like:
– Sourcing process of the standards
– In-house certification of standards, if applicable (including assignment of value)
– Certificate storage
– Storage conditions of the reference standards
– Labeling
– Handling and dispensing
– Inventory
– Expiry and/or recertification date
– Approval process
Reference standards must have a unique number that allows tracing of every delivery if multiple deliveries of the same standard/batch number occur to the test performed.
Quality control reagents
Laboratory chemicals used for testing include reagents, buffers, reference standards, microbial cultures, microbiological indicators, and culture media.
All reagents must be approved after careful assessment and purchased in high purity from authorized suppliers. Reagents should only be used for the purposes for which they were designed.
Certain laboratory reagents are categorized as dangerous commodities, including but not limited to explosives and gases, combustible compounds, peroxides and oxidizing agents, infectious substances and poisons, radioactive materials, and corrosives.
These substances must be protected, and their use should be limited to authorized amounts. After usage, they should be stored in a safe and secure location inside the laboratory.
Proper labeling and traceability are essential when it comes to reagents. All reagents, whether bought from suppliers or made in the lab, must have legible labels with their name, lot or batch number, and vendor information.
More details are required to uniquely identify in-house-created reagents. Labels that identify divisions in reagents should enable us to locate the original source.
It is important to note the date of receipt and the initial opening on the label and other related paperwork, such as the reagent register.
Ensuring that all chemicals and reagents in the laboratory are traceable and accounted for requires maintaining records in accordance with SOPs and regulatory standards.
To ensure that the reagents stay effective until they expire, these must be stored in accordance with their unique requirements.
If usage is to continue after this date, appropriate paperwork and authorization are required.
Expired materials should be discarded promptly. An investigation should be conducted if expired materials are used in error.
Documentation in pharmaceutical quality control
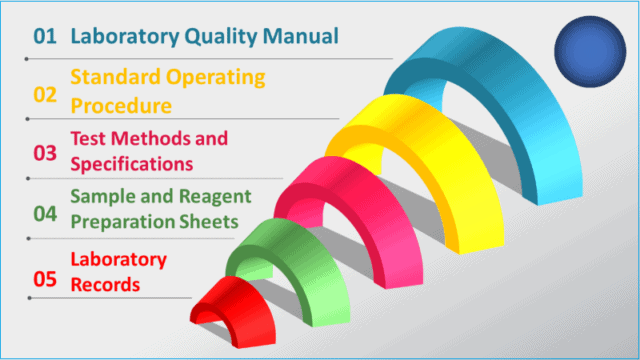
Pharmaceutical quality control documentation is designed as per the following hierarchy:
i. Laboratory quality manuals and policies
ii. Standard operating procedures
iii. Test methods and specifications
iv. Sample and reagent preparation sheets
v. Laboratory records, including the notebook, logbook, register, and fillable forms.
The laboratory quality manual and policies are top-level documents that describe the laboratory’s overall management and organization. They should reflect the requirements under GMP rules.
Standard operating procedures (SOPs) provide more detailed and specific requirements for each laboratory quality element.
For example, SOPs describe how to handle a sample, conduct an audit, release a result from the laboratory, and manage a complaint. SOPs are generally not specific to test methods.
Records must be maintained of the preparation of all media, solutions, reagents, standards, etc., allowing (if applicable) traceability of release (including any release testing results) and traceability of use to arrival in the laboratory.
Where applicable, a documented system describing quarantining and release must exist. This system must also allow traceability of release (including any release testing results) and traceability of use to arrival in the laboratory.
Documents in quality control must follow good documentation practices, as noted in the quality control procedures.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Laboratory logbooks
The quality control laboratory must have log books. Log books are GMP documents and must be designed accordingly. They must also allow space for the person recording information to sign and date.
Typically, log books are required for the following activities:
– Internal receipt and external movement of all test samples
– Disposition of samples
– Use and maintenance of laboratory equipment
– Equipment verification checks
– Column usage
– All cleaning activities must be documented as part of the quality management system in pharmaceutical manufacturing.
– Routine recording of environmental parameters (e.g., temperature, air pressure, etc.)
Laboratory test report
Following is an example of laboratory Assay records that must contain the following:
– Sample description
– Sample unique identifier
– Test method reference
– Unique identifier of all equipment used
– Reference number and expiry date of all chemicals, solutions, reagents, media, reference standards etc. used
– Raw data (e.g., weight weighed, volume measured, calculations, meter readings, observations, etc.)
– Final result
– Printouts, graphs, spectra, etc., from equipment used with unique identifiers linking them to the sample under test.
– Signature (or initials if indicated) of the person who performed the assay and date of activity. Multiple signatures and dates may be required for assays that go over many days and have many steps. All printouts, graphs, spectra, etc., must be signed or initialed by the analyst and dated.
– Provide the signature of the reviewer and the date that the review occurred.
Laboratory management must have a mechanism to track and review the root causes of invalid assays.
Laboratory notebooks
Laboratory notebooks are GMP documents as they provide handwritten evidence of how the sample set is prepared, details of reagents and reference standards used, weighing printouts, testing details, calculations to derive the final results, etc.
Following is a list of details a laboratory notebook should cover:
– Test being performed
– The method used for testing
– Scale ID
– Reagent lot numbers used
– Dilution used
– If more than one dilution is used, indicate which request utilized the particular dilution.
– Sample information
– Name and lot number of material tested
– Weight of sample
– Dilution sequence
– Documentation of raw data
– Enter comments in the designated section.
– Assigned request number for stability samples, enter time point information (e.g., “stability – 30/70/48” which translates to 30ºc/70% humidity/48 months)
– Sample tested by and reviewed by initials
– Instrument ID and calibration dates.
– Test ID and specification number
– Raw data obtained
– Final calculations
Laboratory glassware
Control of glassware is critical for pharmaceutical quality control laboratories, particularly for assays requiring high accuracy.
Class A or equivalent glassware must be used for assays requiring high accuracy, and Class B or equivalent for assays requiring approximate volumes. Assay procedures should indicate the grade of glassware to use.
In a quality control laboratory, procedures must exist that describe the following:
– Cleaning of new glassware before use
– Calibration as applicable
– Uniquely identifying glassware where appropriate
– Checking glassware for damage and disposal
– Cleaning of soiled glassware
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Testing in pharmaceutical quality control
Testing is the most important activity that takes place in a pharmaceutical quality control laboratory.
The laboratory is responsible for testing raw materials against specifications to guarantee their suitability for use in the manufacturing process.
In-process testing assures that the process is not deviating from its defined parameters. If an out-of-specification occurs, it will be detected in the quality control testing.
Finally, finished products are tested in the quality control process to ensure they are manufactured according to the label claim and fit for intended use before being released to the marketplace.
Quality control also engaged in conducting a stability testing campaign to justify the manufactured products’ reliability for patients to take until the declared expiry period.
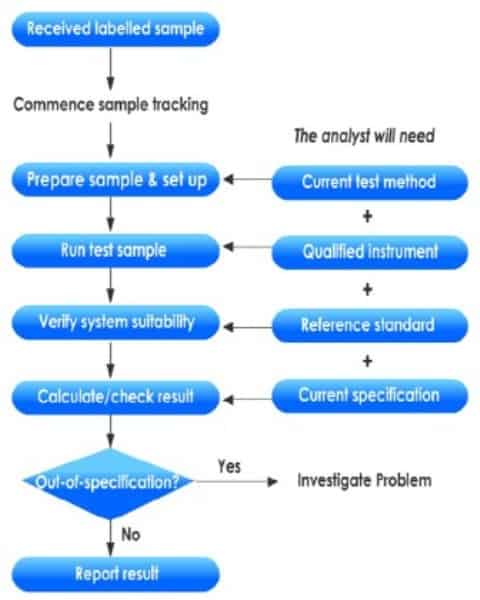
Sample receipt and logging
You can take the following steps while registering the samples in the laboratory and preparing them for testing.
– Enter sample information into the sample logbook.
– Enter the sequential request number.
– Date sample logged.
– Enter sample details as description, batch number, sample type, test required.
– Place the delivered sample in the appropriate test location within the laboratory based on sample type, i.e., raw material, bulk/finished product, and stability sample.
– Water samples or samples requiring refrigeration are to be placed in a sample refrigerator as part of quality control in pharmaceutical settings.
– Controlled samples are placed in the secure storage locker.
Sample testing
– Update sample information in the Logbook: Enter the date the sample was sent to the Laboratory (this indicates the date testing commences).
– Generate protocol according to appropriate SOP.
– Test samples per the appropriate test method maintained within the Laboratory.
– Document raw data in a laboratory information report.
– Tests performed within a defined time period after sample receipt.
– Investigation of sample mix-ups or contamination.
– Sample retention until all tests are completed and there is a determination as to whether a result is valid or invalid.
– Sample destruction or disposal.
Stability studies in QC laboratory
Pharmaceutical products must maintain quality, safety, purity, and efficacy throughout their specified shelf-life until their nominated expiration date.
Stability programs are conducted in quality control testing laboratories to:
– Determine the time during which a product meets registered standards when stored under defined conditions (called accelerated studies)
– To show that the product remains within its expiry specifications throughout its proposed shelf life (called real-time studies)
The difference between release and expiry specifications must, therefore, take into account the results of stability testing.
Purpose of stability studies
Mainly there are two reasons quality control performs stability testing:
i. To provide evidence on how the quality of a drug product varies with time under the influence of a variety of environmental factors, such as temperature, humidity, and light; and
ii. To establish a retest period for the drug product during its shelf life and recommend storage conditions.
Stability studies play a vital role in pharmaceutical quality control, striving to identify the presence of possible degradants in the active pharmaceutical ingredient (API) or drug product matrix.
This proactive approach is crucial as unwanted degradants can be toxic or may interfere with the drug’s effectiveness, ensuring the safety of our consumers.
Instruments used in stability studies
Chromatography (HPLC) is used for API and degradant analysis. Its ability to separate, detect, and quantitate degradants is invaluable.
These assays, known as stability-indicating methods, are highly effective in determining degradant concentrations when used with the appropriate detector sensitivity, column, and mobile phase.
How do you design stability trial protocols?
A protocol is required for each stability study, with a closeout report once the study is completed.
The protocol would contain the following information but not limited to:
– Stability Trial Protocol
– General Product information (i.e., Name, source, manufacturing sites, and date of manufacture of drug substance)
– Dosage form and strength, including formulation
– Composition, type, source, size of container, and closure
– Specifications and test methodology (I.e., Physical appearance, pH, organoleptic, disintegration, chemical, chromatographic, dissolution, degradants, microbiological. total aerobic count, yeast and mold, etc.)
– Study design and plan (i.e., Number of batches, dosage units, sampling points, duration, storage conditions
– Stability data and information (i.e., Data analysis, rules for exclusion of data points, plots, graphics, matrix, etc.
– Results – statistical analysis, estimated expiry date
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Quality control facilities
Pharmaceutical quality control laboratories must maintain detailed maps indicating facilities, utilities, and equipment locations.
Any time a major and permanent change occurs, the map should be revised, and the earlier version will be superseded.
To meet regulatory compliance, instrument rooms, refrigerators, ovens, and wash bays used in the laboratory should be cleaned and maintained according to an approved schedule.
Laboratory instruments should be calibrated upon installation and according to an approved schedule. They should be used within the calibration period or calibrated upon use.
As a good laboratory management practice, the analytical and microbiology laboratories should be built separately into designated areas with limited access and maintained clean and organized.
Practices should be established at the laboratory to ensure laboratory housekeeping is maintained.
Work surfaces in the microbiology laboratory (e.g., benches, bio-safety cabinets, and unidirectional airflow units) should be cleaned, organized, and disinfected before and after use.
Only those disinfectants that effectively clean the expected types of organisms should be chosen.
Laboratory processes (e.g., sterilization) should be validated.
The equipment and utilities (e.g., vacuum, compressed air, gases) should be properly designed, commissioned, and qualified.
Laboratory water systems should also be qualified for their intended use. They should be monitored routinely and suitable for the specified test method or test material preparation, adhering to regulatory requirements.
In addition, the water system in the microbiology laboratory must, at a minimum, meet the quality attributes for purified water.
A documented environmental monitoring program must exist for areas with an official air grade. Data must be trended and reviewed regularly, and alert and action limits must be set.
Procedures must exist for cleaning laboratory rooms, benches, and equipment. Only QA-approved cleaning agents are to be used.
All maintenance and repair programs for laboratory facilities and equipment must be documented where relevant changes must be managed through the change control system.
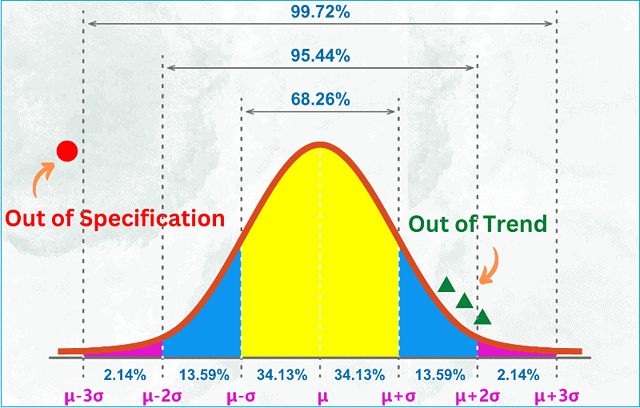
Out-Of-Specification (OOS) Investigations
An out-of-specification (OOS) result lies outside the product specifications.
When you conduct a quality control test on a finished product or raw material, and your test result falls outside of approved, registered, or official specifications, you should assign it an out-of-specification result and commence a rigorous investigation.
For instance, if your assay result appears as 97mg but the approved range from the specification is between 98 mg and 102 mg, your result is out-of-specification.
An out-of-specification (OOS) investigation is required when a result is obtained outside of a specification limit, and the OOS result is deemed confirmed.
You should have a standard operating procedure (SOP) describing the OOS Investigation methodology.
The SOP must include instructions about:
– Timeliness of the investigation.
– You should not dispose of any items, diluted solutions, samples, etc., until the investigation is complete or QA agrees to the disposal.
– Discussions with analysts.
– Examination of raw data, calculations, and transcriptions.
– Controls and / or system suitability criteria.
– Determination that the correct solutions, standards, buffers, media, reagents, etc. were used.
– Clear direction for when a result can be deemed invalid (e.g., the root cause of the OOS result is clearly identified – dilution error) and who can make the decision.
– Clear direction on proceeding with a laboratory investigation protocol where no root cause is identified.
An out-of-specification (OOS) investigation should be performed methodically using a protocol.
Laboratory investigation protocols should always contain the following:
– A description of the OOS
– Initial investigation findings
– The principal behind any re-testing, re-sampling, re-injections, etc.
– The aim of the re-testing, re-sampling, re-injections etc.
– A detailed plan including responsibilities
– Pre-determined results interpretation
– The number of acceptable retests when no root cause is identified.
– Re-testing, re-sampling, re-injections, etc., must be justified and approved as detailed in SOPs.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Sterility testing in quality control laboratory
Sterility testing is critical in pharmaceutical quality control, ensuring that drugs, medical devices, and active pharmaceutical ingredients (APIs) are free from viable microorganisms.
Laboratories must use validated sterility methods, whether compendial or non-compendial, to ensure accurate results. Before implementing any test method, laboratories must demonstrate their performance capability under actual conditions.
Quality control measures extend to the environment where testing occurs, requiring sterility tests to be conducted in controlled environments such as Grade A aseptic processing areas or sterility test isolator systems.
Qualified personnel and facilities are essential for maintaining the integrity of the testing process.
Sampling and testing procedures must be meticulously documented, detailing the number of samples, test methods, equipment used, and test limits.
Reagents and culture media must be purchased from approved suppliers and stored according to specified requirements.
You’ll need thorough investigations before batch/lot disposition in case of test failures.
Continuous training and requalification of personnel are also emphasized to ensure proficiency in sterility testing techniques.
Conclusion
Pharmaceutical quality control is a multifaceted process that ensures medicinal products’ safety, efficacy, and quality.
From collecting and storing samples to rigorous testing procedures and documentation, every step is meticulously designed to uphold regulatory standards and meet patient needs.
Quality control involves testing raw materials and products, as well as establishing and maintaining robust systems and procedures that guarantee consistency and reliability throughout the manufacturing process.
It encompasses various activities such as personnel training, equipment qualification, test method validation, and management of reference standards and reagents.
Personnel training and qualification are not just a formality but a necessity.
GLP training ensures that every individual involved in the quality control process is not just competent but highly capable of performing their duties effectively.
Training and requalification foster continuous quality improvements and stay abreast with evolving regulations and industry best practices, instilling confidence in the process.
Furthermore, the quality control program also encompasses environmental monitoring, facility management, and adherence to strict protocols for handling out-of-specification results.
These measures are critical in maintaining a clean and controlled environment conducive to accurate testing and reliable results.
By strictly adhering to stringent quality control practices, pharmaceutical companies can guarantee that their products consistently meet the highest safety, purity, and effectiveness standards, thereby making a significant contribution to public health and well-being.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.