General Guidance on Pharmaceutical Deviation Management
- Published on: Dec 16, 2020
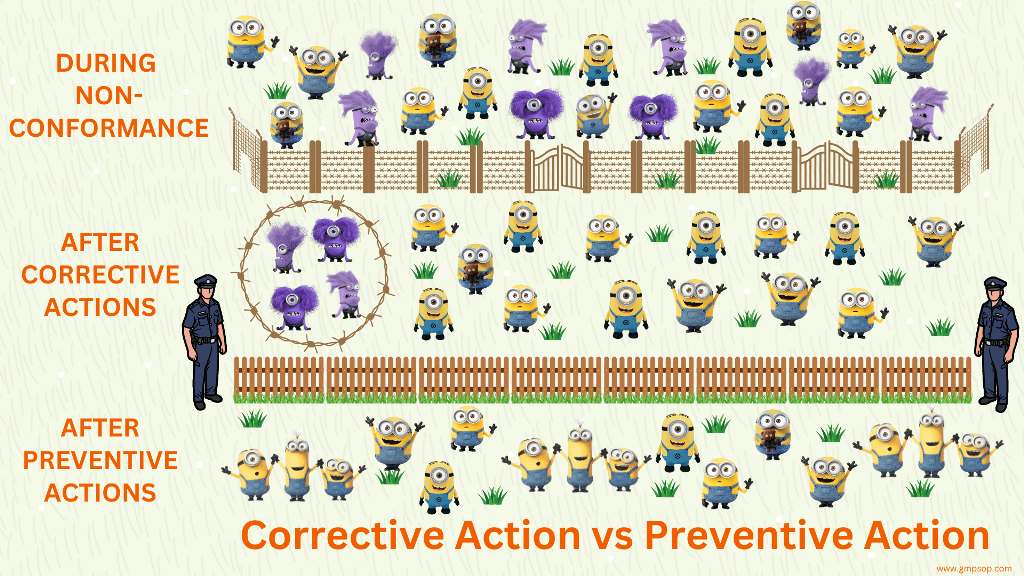
A deviation is a departure from standard procedures or specifications resulting in non-conforming material &/or processes, or where there have been unusual or unexplained events which have the potential to impact on product quality, system integrity or personal safety. For compliance to GMP and the sake of continuous improvement, these deviations are recorded in real time, investigated with root cause analysis and corrective and preventative actions are implemented.
Types of Deviations
Following is a list of areas where deviations can be observed:
- Production Deviation – usually raised during the manufacture of a Batch Production.
- EHS Deviation – Raised due to a “EHS Hazard”.
- Quality Improvement Deviation – may be raised if a potential weakness has been identified, and the implementation will require project approval.
- Environmental Monitoring Deviation – Out of specification environmental monitoring test results
- Audit Deviation – Raised to flag non-conformance identified during internal, external, supplier or corporate audits.
- Customer Service Deviation – Raised to track implementation measures related to customer complaints.
- Technical Deviation – deviation can be raised for validation discrepancies, ie. Manufacturing Instruction modification etc.
- House-Keeping Deviation – raised as a result of a housekeeping audit.
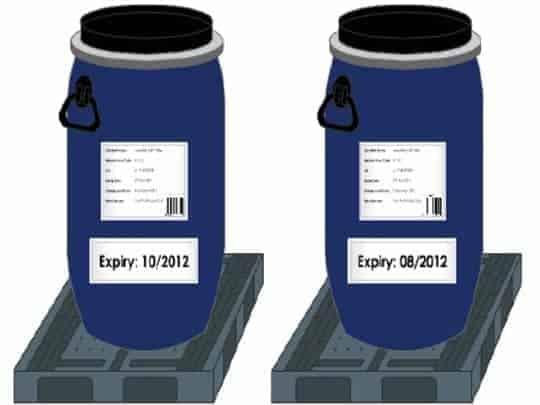
- Material Complaint – raised to document any issues with regards to non-conforming, superseded or obsolete raw materials/components, packaging, or imported finished goods.
- System Routing Deviation – raised to track changes made to BOM (Bill of materials) as a result of an Artwork change.
The purpose of this article is to establish the expected compliance standard and approach to investigating and resolving both planned and unplanned deviations at a GMP sites
210 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Planned Deviation
A planned deviation is required when a process is planned to deviate from a current operational SOP, for a specific batch or batches or a predefined time period.
A system must exist that logs and tracks planned deviations.
A planned deviation is only considered as an exception.
A planned deviation must be preapproved by the requesting department head and a QA Manager prior to implementation.
The QA Manager may ask advice from other department heads such as Engineering or Development prior to approval.
- Planned deviations must cover
- Compliance to GMP
- Regulatory Compliance
- Product risk
- Impact on other products
- Impacted markets
- Consideration of notification to other affiliates or third parties
All planned deviations should contain the following details:
- Material / Product Description and code
- Defined batch numbers or anticipated timeframe
- Current procedure and any other associated documents
- Proposed deviation
- Reason for deviation and justification
- Risk assessment if appropriate
The QA Manager must assess product impact.
The QA Manager must stipulate any corrective actions.
The planned deviation can be closed once approved and any corrective actions are completed.
A copy of the approved planned deviation must be available in the batch record.
Unplanned Deviations
An unplanned deviation may occur at any stage of the manufacturing, packaging, testing, holding or storage process.
The deviation may be a result of system failure, equipment breakdown or manual error.
If the deviation has any impact on product quality, purity or strength QA should be notified immediately.
An investigation into the incident is completed by the relevant department. A SOP should exist to aid in completing a detailed investigation. The following should be considered when investigating deviations:
- Review of all documentation: Log books, SOP’s, batch records etc.
- Examination of raw data
- Interview with involved personnel
- Review of past experience
- Impact on other products
A root cause should be established with consideration given to:
- Materials
- Method
- Personnel
- Equipment
- Environment
The possible root cause should be reported based on the evaluation and conclusion.
Where appropriate immediate action should be taken.
Any resultant CAPA should be executed and effectiveness assessed prior to deviation closure.
Product Out of Specification Results
An Out of Specification (OOS) investigation is required when a product test result is outside the specification limits.
A SOP must exist that describes the methodology for performing an OOS Investigation, the SOP must include instructions in relation to:
- Timeliness of the investigation.
- Not disposing of any items, diluted solutions, samples etc. until the investigation is complete or QA agree to the disposal.
- Discussions with analysts.
- Examination of raw data, calculations and transcriptions.
- Controls and / or system suitability criteria.
- Determination that the correct solutions, standards, buffers, media, reagents etc. were used.
- Clear direction for when a result can be deemed invalid (e.g. root cause of OOS result clearly identified – dilution error, control test failure.
- Only QA can invalidate a test result
- Clear direction on how to proceed with a Laboratory Investigation Protocol where no root cause is identified.
Laboratory Investigation Protocol’s should always contain:
– A description of the OOS
– Initial investigation findings
– The principal behind any re-testing, re-sampling, re-injections etc.
– The aim of the re-testing, re-sampling, re-injections etc.
– A detailed plan including responsibilities
– Pre-determined results interpretation
The number of acceptable retests when no root cause is identified.
All investigations must be clearly documented and attached to the Deviation Report.
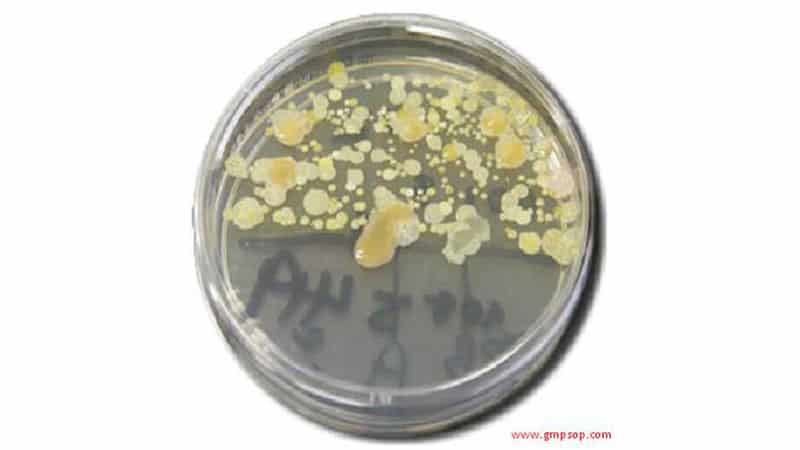
Environmental Monitoring Out of Specification
A SOP must exist that describes the methodology for investigating environmental monitoring excursions that may impact product quality. The procedure must consider:
- Timeliness of the investigation.
- Not disposing of any items, including sample plates and isolates. until the investigation is complete or QA agree to the disposal.
- Discussions with analysts.
- Discussions with operators
- Examination of room activity logs and batch records.
- Determination that the media plates were used correctly in the correct locations.
- Prior cleaning and fumigation
- Clear direction on how to report investigation findings.
- Reports should include:
– History of the room
– Media fill data
– Room pressure profile
– Interventions
– All environmental test results for the room
– Sterility testing of product if it was filled in the area at the time of excursion
– Operator Data including:
– Operator Training Records
– Operators gowning qualification status and trend
- Operator trend of environment monitoring in affected room including Settle plates, Contact plates, Viable air sampling and Non-viable particulates monitoring
- Assessment of potential causes for each deviation
Operator trend of environment monitoring in affected room including Settle plates, Contact plates, Viable air sampling and Non-viable particulates monitoring. All investigations must be clearly documented and attached to the Deviation Report.
The QA Manager must make an assessment based on the report as to product impact and likelihood of contamination, before the deviation can be closed.
Water testing results Out of Specification
If an alert level for microbial or chemical analysis is exceed for purified water or water for injection a deviation must be raised and QA notified.
All available data will be analysed and a report generated that includes:
- Trended data and all results for the same day
- Identification of the contaminant
- TOC (Total Organic Carbon) monitoring data, conductivity, temperature and pressure
– The integrity of the system should be confirmed.
– All equipment should be checked for integrity.
– A risk assessment should be conducted.
– A QA Manager will assess the data and determine if the water is fit for continued operation or if corrective action is required.
CAPA Management
A SOP must exist that outlines the process of CAPA management and tracking. The SOP must include:
- Clear description of the non-conformance requiring correction
- The investigation that determines the action to be taken
- Timelines for closure
- Person responsible for correction
- QA approval
- Details of resolution
- Effectiveness Check
- Tracking mechanism to ensure all items are addressed
Trending Deviations
Status of deviations and CAPA should be reported to local senior Management meetings. Reporting requirements should be detailed in a SOP and include:
- Number of open deviations
- Number and type of open planned deviations
- Classification of deviations
- Number of overdue CAPA and plan for remediation
- Details of any ineffective corrective action
Time frames for responding to deviation investigations
Critical deviations that pose a risk to product and animals must be escalated to senior management within 1 day of identification. An initial investigation is required within 3 days, with closure in 30 days. An interim report may be issued at 30 days if closure is not possible.
Major deviations, that if not resolved may pose a risk to product quality should be closed in 30 days. An interim report may be issued at 30 days if closure is not possible.
Minor deviations which are unlikely to pose a risk to product quality should be closed in 60 days or an interim report must be provided.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.