How to conduct GMP training for employee
- Kazi
- Last modified: March 15, 2025
A successful GMP training for employees ensures that all staff will receive adequate, relevant and documented training to enable them to perform their assigned functions safely and comply with applicable guidelines/regulations.
It is a GMP/GLP requirement that all personnel be trained in the work appropriate to their role.
FDA-regulated industries should prepare GMP Training policies and procedures for their employees outlining training approaches, scope, responsibilities and overall training system.
There should be a clear process to establish training needs, provide training, and evaluate, record, and reward archives as required by the local regulatory authorities.
Table of Contents
Types of GMP training for employees
GMP-regulated businesses carefully determine what type of training is practical and reasonable for their staff. Following is a list of common GMP training that most GMP sites are accustomed:
- Induction training
- Skills training
- Good Manufacturing Practices (GMP) training
- Good Laboratory Practices (GLP) training
- Quarantine requirement training
- Gowning qualification training
- Work, Health and Safety training
- External certification training
- Permit to Work systems
- Personal Protective Equipment (PPE)
- Bio-Security
- Permit to Work systems
- Auditor training
- Hazard/Incident Reporting and Investigation
Out of the above training, induction, skill and Work, Health & Safety training are applied to all employees, contractors (including cleaning staff) and casual staff.
GMP/GLP and PPE training are applied to all employees, contractors and casual staff working in a GMP environment, including manufacturing, packaging, labelling, distribution, QA, QC, development, validation, engineering, IT staff and anyone whose activities could affect product quality.
Gowning qualification training applies to all staff working in cleanroom processing areas on top of the training regime mentioned above.
Quarantine and bio-security training apply to employees working in biological processing sites. Permit to Work training is required for engineering and project staff.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Responsibilities for a successful GMP training program
A GMP training for employee program should start at the top of an organisation and flow directly to the bottom.
It can be effective to create a training task force or individual with support from senior management, who coordinates training and is responsible for its implementation.
Employees:
All employees and contractors are responsible for the following:
– Know their training requirements.
– Actively participate in training and assessment and inform the trainer if they do not understand any of the material.
– Sign a training record indicating that they believe they are competent to conduct the work they have been trained in or (where the training is knowledge training) that they understand the material they have been trained in.
– Could you discuss their individual training and development needs with their supervisor and/or manager in the first instance? Employees may request initial information from the human resources department.
– Carry out agreed development plans.
– Maintain a copy of their training records in their work area – in the folder provided by the training system administrator.
Quality Assurance Manager:
Typically, the QA Manager is responsible for approving the design and implementation of the training system in collaboration with the human resources department. The role is responsible for ensuring that:
– The Training System is GxP compliant.
– System reporting complies with KPI requirements for the business.
– The training system is audited annually.
– Approves the GMP/GLP training schedule and training plans for each role.
Work, Health and Safety:
The WH&S manager or specialist proposes and conducts a safety and environment training program that meets the WH&S and Environment compliance requirements as regulated by the local authorities. The team maintains records of all safety and environment-related training.
Department Managers and Supervisors:
Department Managers and Supervisors are responsible for:
– Employees are trained in the work of their role in an agreed timeframe. This includes training staff who are filling a role in a temporary capacity.
– New employees and contractors undergo GMP induction training within the first week of employment.
– Contractors undergo induction and other training relevant to their role in a timely manner.
– Training requirements for each role are identified, documented, approved and kept current.
– A plan is in place to ensure that training and development requirements for each employee (and contractor, where appropriate) are met on time.
Training Administrator:
The Training Administrator is responsible to:
– Ensure that training matrices and records are kept current, secure and accessible. This includes ensuring that references to the version of a given document align with the corresponding entry on the Controlled Document Index.
– Report training progress to managers, supervisors and employees.
– Ensure hard copy training records, accreditation certificates, and GMP training certificates are retained and appropriately filed.
– Maintain a current list of trained and accredited staff.
– Audit the system periodically to ensure it complies with the company procedure.
– Provide supervisors/managers with draft training matrices and training plans for new roles.
– Provide monthly training status reports to the business.
Human Resource Manager:
The HR Manager is responsible to:
– Provide assistance to management in planning, coordinating and implementing relevant, cost-effective training and development activities for all non-QA, GMP and WH&S-related training.
– Notify the training administrator of all new staff appointments and cessation of employment. This includes permanent and casual staff.
– Forward induction program information and records (including GMP and safety induction) to a new employee’s manager at the commencement of employment.
– Archive the completed training into employee’s personal files. Archive the training record for defined period after the employee has separated from business.
GMP Trainers:
GMP Trainers are responsible to:
– Deliver the training in the most effective manner.
– Ensure that after training, training records are signed – by each trainee and the trainer.
– Forward the original training record to the Training Administrator for record-keeping and archiving.
GMP training for employees - essential steps
The development, implementation and execution of employees’ GMP training can vary depending on business operations and respective regulatory expectations. In principle, the GMP training program can be built around the following steps with clear objectives and deliverables.
The training program should start with training requirements, planning and design, delivery, assessment, record keeping and archiving stages.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
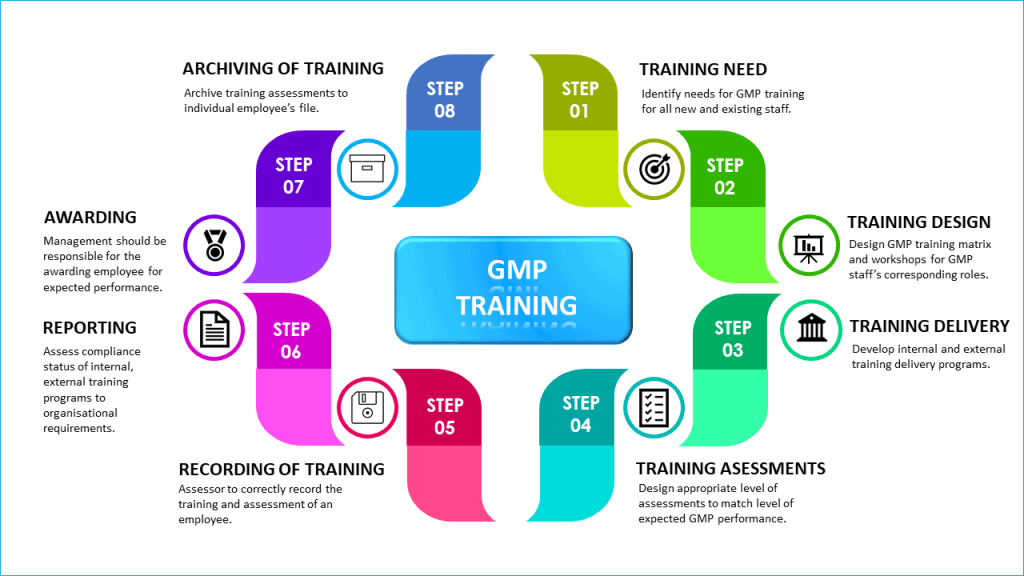
Step 1: Training need
The senior managers and subject matter experts should conduct the following analysis to identify employee training needs correctly.
– Request feedback from the department managers
– Review feedback from employee development need
– Evaluate internal audit findings
– Review FDA or customer audit findings
– Review policies and plans that need to be complied against
– Review compliance with in-house and regulatory requirements
– Reviewing GMP breaches and incidents to assess trends
– Could you identify organisational opportunities for training?
Step 2: Training design
At the step of designing the training program, the management and subject matter experts should ask the following questions:
– Who is to be trained?
– What are the training topics or subject matter?
– From where will training materials come?
– Who would be the trainers?
– Where to conduct training?
– What is the cost of training?
– What is the duration of training?
– What are the schedules and priorities? Etc.
During the design phase, the team should draw a skills training matrix for each role, listing the skills, and reference training courses against each employee who must be trained.
In the skills training matrix, the timeframe should be set by which employee must complete their training. The skills matrix may refer to written procedures, training modules or other training resources.
GMP Points can be designed to be earned by the employees through attendance at internal and external GMP Training courses, workshops, certifications, etc.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Step 3: Training delivery
There are multiple ways training can be delivered. Some of those are listed in the beginning.
However, types of training can vary from business to business and their requirements respectively. Some of the common training are listed below:
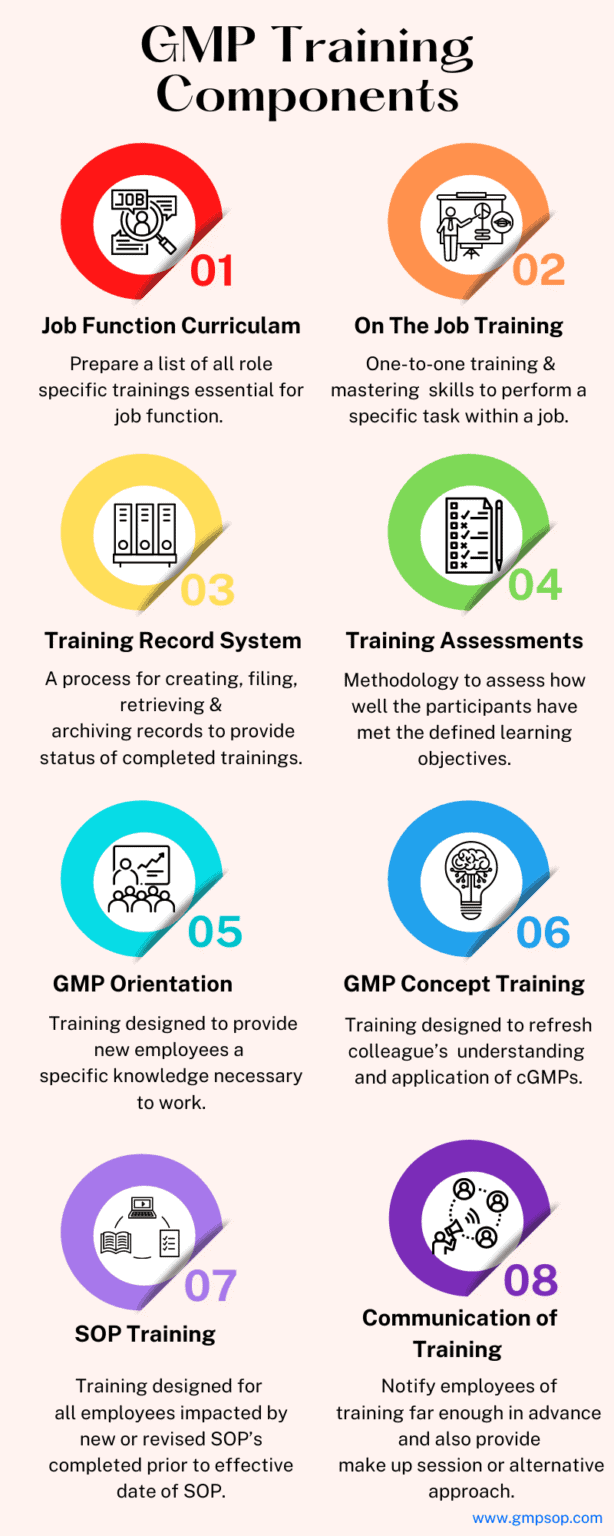
i. Job function curriculum
A list of all training must be completed and kept current for each person in that job function.
Each item on the Job Function Curriculum must be completed as specified unless there is a documented exemption for an item based on a combination of their previous education, training, or
experience. The curriculum must include achievable targets that, once completed, allow the person to work on a GMP task without direct supervision.
ii. On-the-job training
The one-to-one process of providing and mastering knowledge and skills to perform a specific task within a job. Training should include specific criteria to be met to show that competence has been achieved in the task.
iii. GMP orientation
Training is designed to provide new employees with the fundamental knowledge necessary to work in a specific area. For example, regulations, industry history, health and hygiene, documentation practices, GMP values and other site-specific information.
Typical areas to cover during new employee orientation include but are limited to the following:
a. Facility Overview:
– General layout of the facility
– Flow of materials and people
– Controlled access areas
– Departmental organisation
b. Product Overview:
– Controlled drugs
– Dosage forms
– GMP
– Applicable regulations
– Introduction to the FDA
c. Documentation:
– Requirements
– Logs
– Forms
– Rules for corrections
d. General Operating Procedures
e. General SOPs in the specific areas
f. Contamination Control:
– Handling toxic compounds
– Handling infectious compounds
– Gowning
g. Safety
– Alarms
– Evacuation/emergency procedures (i.e. fire, flood, weather, earthquake)
– Material Safety Data Sheets (MSDS)
h. Operational Requirements
– Aseptic
– Controlled environment
– Hardhats
– Lab coats
– Personal Protective Equipment (PPE)
– Reconciliation
– Rejected material
– Quarantined material
k. Equipment
– Operation
– Safety
– Calibration
iv. GMP concept training
Training designed to refresh colleague’s understanding and application of cGMPs.
The courses should be designed to promote learning and improve colleague’s focus on cGMPs, regardless of their specific job function.
v. Standard operating procedure (SOP) training
Training designed for all employees impacted by new or revised SOP’s.
This training must be completed before the SOP’s effective date, ensuring that the employee has been fully versed on all changes affecting their job function.
vi. Read and understand training
In this type of training, employees read the document (often a procedure) and sign training documentation stating they read and understand the document.
Usually, there are no tests on the document.
vi. Self-training
Some employees take the initiative and pursue additional training, which should be encouraged by the business. These are often integral to the employee development part of the performance review program.
Some commonly recognised self-training include:
– Membership in professional societies
– Training outside the company to increase understanding or knowledge on a job-related topic
– Visits to other companies
– Publications
– Magazines & Journal subscriptions
– Manuals/booklets
– Text Books, etc.
Step 4: Training assessments
An instrument or methodology should be in place to gather feedback to improve the training process.
For example, a survey questionnaire should be requested from the participants that measures training effectiveness relative to satisfaction.
An instrument or methodology should be introduced to measure learning assessment. The methodology may include:
Measurement to assess how well the participants have met the defined learning objectives and or links to business results.
– Measure trainee recall via written, verbal, computer-based assessments, case studies, simulations or demonstrations
– Measure trainee performance via direct observation, feedback from others, performance attributes or parameters.
– Measure the impact of training on business performance by evaluating business results, metrics or data.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Step 5: Recording of training
A formal process for creating, filing, retrieving, and archiving training records that will track and report the status of completed and required training should be developed.
Training records or reports must be readily available to substantiate that all employees performing GMP-related functions have completed the training requirements.
The training officer and the department manager must store the training and assessment records in individual employee’s files with the following details:
– Title of course and course code
– Date/s of course
– Trainer’s name
– Name of employees who attended
– Assessment results, including the assessor’s signature
Step 6: Reporting of training
Typically, it is the role of the training officer or administrator to provide training reports that include the:
– Assess compliance of internal training programs to organisational requirements.
– Provide reports on current GMP compliance status for training.
– Maintain training records for QA.
– Compile performance KPIs (Key Performance Indicators) as required.
– Facilitate annual review of QA training requirements.
The training officer should also provide reports to management periodically with the following data:
– No. trainees trained on all procedures.
– No. trainees with outstanding training.
– A list of outstanding training for each trainee.
– A list of outstanding gowning qualifications.
– A list of outstanding Aseptic Practices Training.
Step 7: Employee awarding
The responsible training office should update the skills training matrix once the trainings are completed, assessed and recorded.
Employees are given cumulative scores on the matrix for a period of typically one year based on their assessment performance.
It is important for the senior managers and department managers to recognize the high-scoring employees for their persistent hard work and reward them appropriately for dedication and commitment.
Step 8: Archiving of training
All training records clearly showing the employee name, role, course title, completion date, trainee signature, assessor name and signature, assessment score, etc., should be archived after an employee departs the workplace for a defined number of years as required by the local regulatory authority.
Personnel data should be securely kept using physical means such as compact discs or electronically by scanning the records and uploading them into cloud-based storage.
Conclusion
GMP training for employees is a process that requires continuous attention from senior management top down to floor employees.
The system requires a team effort, and success warrants close oversight from the responsible personnel. Participation in GMP training from all levels of employees is mandatory.
So long the GMP training process is carefully planned, constructed, executed, and reported, and employees are rewarded, the business could reap significant benefits over time by complying with regulatory requirements, consistently producing quality products, prolific sales revenue and high employee morale.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.