
Line clearance procedure and reconciliation in GMP
- Kazi
- Last modified: March 13, 2025
What is line clearance?
Line clearance in pharmaceutical industry is a set of activities or checks performed just before an operation can commence.
Line clearance ensures the processing line is free of any irrelevant products, components, and documentation that could be left accidentally from an earlier batch.
Line clearance is the first step in a process, and it is to be done before a line opening.
Line opening ensures correct products, components, and documentation are brought into the processing line so the operation can commence without error.
You can commence the processing line after a successful line clearance, setup, and opening.
The final step in the sequence is line cleaning, which ensures no raw material, components, or processing records are left behind on the line to avoid mix-ups with the next lot.
Table of Contents
Key Takeaways
- Line clearance in pharmaceutical industry involves physical segregation of processing lines, preventing product and documentation mix-ups.
- Regulatory guidelines require written procedures and a checklist for performing line clearance. The main idea is to separate processing lines, clean work areas, and equipment, remove previous products and waste, and reconcile materials from prior batches.
- Line clearance involves verifying that no foreign object from the earlier batch is present on the line. Examples include leftover drug materials, cartons, leaflets, bottles, batch records, label reels, dust, and fragments.
- The line clearance checklist for a packing line should include checking for label jams or leftover labels, inspecting beneath the packaging machine for fallen materials, and testing barcode readers and control sensors before the start of the packaging process.
- Line setup is followed by line clearance. Line setup ensures the line is ready for a new packaging operation. It involves verifying the line is cleared, allowing new materials, confirming that all bulk containers are approved, checking printed matter against batch records, and labeling the line with the product name and strength.
- A line opening is performed after line clearance and setup. It ensures all components, machine settings, batch numbers, and other details are correct before starting production. Line opening involves using a checklist alongside batch instruction, sampling each component, and attaching it to batch records.
- It is a GMP rule that two authorized personnel should conduct line clearance, setup, and opening activities. They should verify and sign off on each other's checks. All steps must be completed and signed before a new batch production can begin.
- GMP rules around line clearance procedures require various online checks. These checks confirm the accuracy of bulk materials, product appearance, equipment ID, correct labels, container count, label adhesion, cap tightness, fill volume, seal integrity, etc.
- Barcode readers are sometimes used in the packaging line as online control. Barcodes ensure the accuracy of packaging operations. If a barcode reader is faulty, it must be repaired, re-validated, and returned to the line.
- Production run charts and control charts are popular for monitoring the performance of manufacturing processes. They track the actual values against the target and check if the outputs are falling within the specification range. Adjustments are required if the results fall outside these limits, and the batch may be quarantined until the investigation is completed and the root cause is assigned.
- Reconciliation ensures that all materials are accounted for, preventing mix-ups. It's a key GMP control, especially for drug products and printed matter. It involves comparing the issued quantity with what's used, damaged, returned, or wasted. Discrepancies in reconciliation can result from material loss, foreign contamination, or miscounting.
- While yield focuses on product output, reconciliation addresses material losses or gains.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Why line clearance is important in pharmaceuticals?
Many products are generally processed at once in a busy pharmaceutical packing hall, sometimes in adjacent packing lines.
Unless specific GMP controls are in place (e.g., line clearance, line cleaning), a rogue item of product or printed material from an earlier packing line may inevitably end up on a different product – a typical case of component mix-up.
A mix-up may not be detected after the batch is made, which may contaminate the product, harm patients, and lead to a potential recall.
Proper line segregation involves physically separating different lots of starting materials, labeling components, and printing matters, many of which look similar.
Critical controls in GMP include physically segregating the processing lines, double-checking the line when new printed matter arrives, and recording the activities in a line clearance checklist.
Line clearance guidelines FDA
US FDA CFR 211, Sec. 211.130 Packaging and labeling operations quotes,
– There shall be written procedures designed to ensure that correct labels, labeling, and packaging materials are used for drug products; such written procedures shall be followed. These procedures shall incorporate the following features:
– Prevention of mix-ups and cross-contamination by physical or spatial separation from operations on other drug products.
Other international GMP regulations quoted,
– When setting up a program for packaging operations, particular attention should be given to minimizing the risk of cross-contamination, mix-ups, or substitutions.
Different products should not be packaged in proximity unless physical segregation exists.
– Before packaging operations begin, steps should be taken to ensure that the work area, packaging lines, printing machines, and other equipment are clean and free from any products, materials, or documents previously used if these are not required for the current operation.
You’ll need to perform line clearance according to an appropriate checklist.
Line clearances are used in the labeling and packaging area as another control to prevent mix-up of product; the following steps must be conducted and verified before the start of packaging and labeling operation:
– Clean the area and machines,
– Remove all previous products and machines from the area.
– Remove all waste from the area and machines.
– Reconcile all printed material and products from the previous batch.
Once you complete the above steps, it is important to inspect the area and machines and find that they are free of all previous products.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How do you perform line clearance using a checklist?
Before filling and labeling, you should thoroughly examine the filling line using a standard operating procedure. This is typically done through a line clearance checklist.
Line clearance should ensure that all materials, products, labels, and records from previous operations have been removed.
The person responsible should initial the batch record to show that a clearance check has been carried out.
The line should be clearly labeled to show the product and its strength to be packaged. Label counters or code readers should be tested to verify that they work correctly.
Labeling and packaging material should be carefully checked to match the descriptions in the batch packaging records.
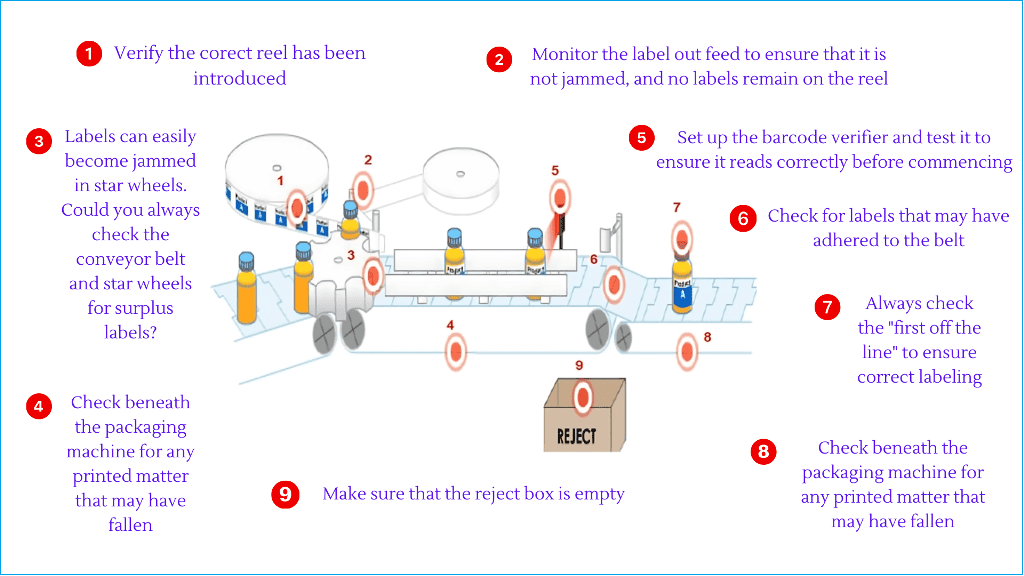
Please look at the labeling operation in the picture below.
In the sequence, filled bottles are entered into a packing line, labeled, and passed through a check weigher and automated sensor. If the labeled bottles pass the control tests, these are then allowed to be packed into a shipper.
In this labeling sequence, we have highlighted nine points to be included in the line clearance checklist.
The points are:
1. Could you verify that the correct reel has been introduced?
2. Monitor the label-out feed to ensure that it is not jammed, and no labels remain on the reel.
3. Labels can easily become jammed in star wheels. Could you always check the conveyor belt and star wheels for surplus labels?
4. Check beneath the packaging machine for any printed matter that may have fallen.
5. Set up the barcode verifier and test it to ensure it reads correctly before commencing.
6. Check for labels that may have adhered to the belt.
7. Always check the “first off the line” to ensure correct labeling.
8. Check beneath the packaging machine for any printed matter that may have fallen.
9. Make sure that the reject box is empty.
How would you conduct a line setup?
Line setup is performed just after the line clearance is passed.
Before a new packaging and labeling operation can begin, you should perform a line setup which includes:
– Verifying that the line has been cleared
– Assembling the material needed for the new operation
– Verifying that each container of bulk material is approved for use
– Verifying that all printed matter conforms to the descriptions in the batch record
– Labeling the line with the product name and strength
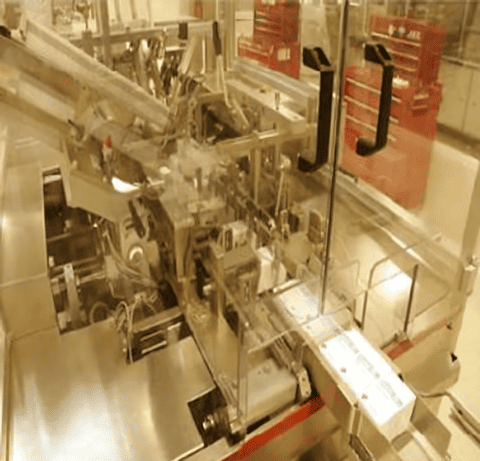
GMP rules require that lines be physically separated to prevent packaging mix-ups. This generally means partitioning between adjacent production lines.
Note the glass wall between the two packaging lines in the processing floor below.
This prevents materials from being inadvertently mixed up between the lines and operators from passing materials back and forth from line to line.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How do you perform a line opening?
After line clearance and setup are completed and signed, a line opening is carried out.
Line clearance ensures all related components, machine settings, batch number, expiry date, date of manufacture (if required), and information boards are all correct and complete before commencing the next operation.
The layout of the line opening checklist must be used in conjunction with batch instruction documents. It will ensure that the components are correct.
A sample of each component is to be taken and attached to the appropriate sample sheet form.
Each sample is to be entered in the relevant section of batch instruction documents. Upon confirmation of correct details, each person carrying out line opening ticks, signs, and dates the sample.
As the process is carried out, the line opening form must be completed and signed by two authorized persons counter-checking each other’s work.
The line opening checklist and other batch documentation must be completed before production starts.
Suppose an additional issue of components is brought into the area during a process run. In that case, the components must be checked against the batch documentation and entered into the batch instruction documents.
A sample of all printed materials must be signed and attached to the sample sheet.
During a line opening, the product must be checked for correct details (batch, expiry, embossing, and product details).
How do you perform a line cleaning?
After an operation, an authorized person must perform the line cleaning using an approved line cleaning form.
You should employ multiple trained and assessed team members to clean and clear the line.
The operator conducting the line cleaning is responsible for ensuring that the tasks have been performed and that the line cleaning checklist has been signed.
During line cleaning, some guards and parts may be left open or removed to complete the line clearance for the next operation.
Following the completion of an operation, the product and process documents must be removed from the area, together with any remaining materials from that operation.
Please remember the line and the machine must be cleaned in accordance with the relevant SOP for the process.
The cleaning checklist should not be signed until the cleaning and housekeeping standards are met, as documented in relevant SOPs for each piece of equipment.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Automated controls in line clearance?
Where multiple production lines operate simultaneously, a common occurrence is misdirected packaging materials.
Besides the normal automated quality controls on the production equipment, misdirection of packaging materials can be minimized by assigning a color code to everything associated with particular production lines (e.g., the “blue” line, the “red” line).
A great practice is color-coding the containers or totes used to issue packaging and labeling to the production line.
Hence, a trained operator can notice and react to the presence of wrongly colored tote bins.
This simple procedure provides an extra measure of quality control.
1. Online Controls
Reliable controls such as barcode readers are a popular practice in packaging operations.
Removing or turning off a barcode reader removes an important packaging control measure.
At the same time, if a barcode reader is misreading regularly, that is also not acceptable.
To prevent such waste, the barcode reader should be repaired, returned to the line, and re-validated.
US FDA CFR 211, Sec. 211.130 Packaging and labeling operations quoted that,
“Examination of packaging and labeling materials for suitability and correctness before packaging operations, and documentation of such examination in the batch production record”.
During the packaging and labeling operation, GMP rules specify certain necessary online (in-process) controls.
Online controls should include the following checks:
– The bulk material and printed matter.
– The product’s appearance (e.g., chipped labels, particles in sterile injectable).
– The equipment used (including the label verifier).
– The count or measure of the filled container.
– The label appearance and adhesion coding.
– The tightness of the cap.
– The seal integrity of the packs.
When these checks are done by production staff, the check should be audited by the Quality Department.
2. Checking fill volume and seal integrity
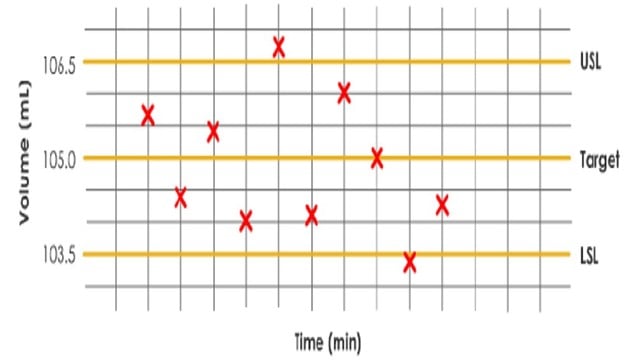
i. Production run charts
Run charts are often used to monitor manufacturing processes. Please check the production run chart presented above:
– Target= 105.0ml
– Lower specification limit (LSL) = 103.5ml
– Upper specification limit (USL) = 106.5mL
– Actual progressive volumes dispensed over time
From the diagram, some points appear above and below the upper and lower specification limits.
This means the process would have to be adjusted, and the batch would need to be quarantined.
The run chart does not indicate whether the process has consistent (predictable) variation or is “out of control.”
You can play with a simple Run chart and Control chart calculator and learn how to determine out-of-specification and out-of-trend events.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
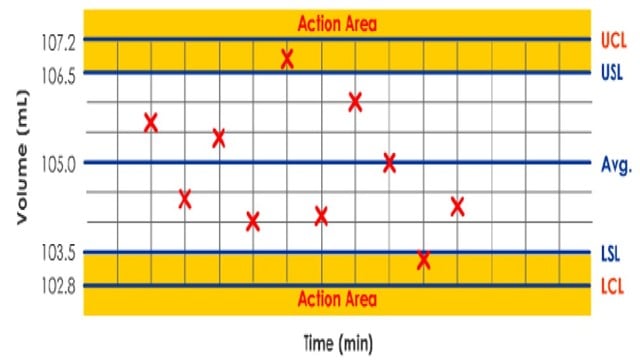
ii. Control Charts
Packaging processes can be monitored using either run charts or control charts.
A control chart is more complex but provides better control over the process.
Specific rules also govern when to adjust the process and when to leave it alone. These rules are not solely based on meeting packaging specifications.
Control charts are established based on statistical theory regarding process variation.
If your company uses charting to control processes, please ensure that you have been trained in its use and interpretation.
Control charts are statistical tools used to determine whether a process can perform within specification limits.
They also provide rules for changing or adjusting the process should it get “out of control.”
A control chart is bounded by upper and lower control limits (UCL and LCL) calculated from the process’s natural variation.
If these limits are within the specification limits, the process is termed “capable.”
In the following production control chart example, the control limits are wider than the specifications’ limits, so the process is classified as “not capable.”
Because the process isn’t capable, action is required to bring the process back into control. If no action is taken, the manufacturing process should not be used.
You can play with a simple Run chart and Control chart calculator and learn how to determine out-of-specification and out-of-trend events.
What is reconciliation in pharmaceuticals?
Pharmaceutical reconciliation ensures that all materials have been accounted for and no mix-up has occurred.
Reconciliation of printed matter is one of the key GMP packaging controls. The reconciliations indicate if any printed matter is missing or not accounted for.
Reconciliation is done on printed, coded components and the finished product.
Reconciliation is essential when a batch undergoes a rework procedure to fix quality defects. It is an integral part of the rework protocol.
Reconciliation should be performed at the end of each major operation step, especially if the components are moved from one location to another. The final reconciliation should cover the whole process.
The reconciliation calculation should be based on real figures. Estimate is only allowed in relation to materials that can be “lost” during the process.
In a reconciliation, the quantify issued to (a known or verified count) should be compared with the sum of:
– The quantity used for a good product.
– The quantity used for the rejected product.
– The quantity used for samples.
– The quantity used in documentation.
– The quantity damaged.
– The quantity returned.
Every single measure is a count, not a calculation.
An unsatisfactory reconciliation can indicate a loss of material, a gain of foreign material, a miscounting, or bad arithmetic.
Regulatory guidelines for reconciliation
US FDA CFR 211, Sec. 211.125 Labeling issuance quoted that,
(a) Strict control shall be exercised over labeling issued for use in drug product labeling operations.
(b) Labeling materials issued for a batch shall be carefully examined for identity and conformity to the labeling specified in the master or batch production records.
(c) Procedures shall be used to reconcile the quantities of labeling issued, used, and returned and shall require evaluation of discrepancies between the quantity of drug product finished and the quantity of labeling Issued when such discrepancies are outside narrow preset limits based on historical operating data.
As you know, such discrepancies will be investigated following 211.192. Labeling reconciliation is waived for cut or roll labeling if a 100% examination for correct labeling is performed per 211.122(g)(2).
Yield Vs. reconciliation
Yield and reconciliation are two ways of looking at material balance.
Yield focuses on acceptable product outputs and is usually used for production processes. For example, a production line’s yield is 100%, which means the line is generating the expected outcome at 100%.
Reconciliation focuses on losses or apparent gains of materials and is usually used for labeling and packaging operations.
Reconciliation compares the amount of material going into a process with the amount coming out.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How do we perform reconciliation?
At the end of each specific batch or part-batch packing, the machine operator will tally the printed components and products left, such as labels, cartons, leaflets, tablets, etc.
These components will be reconciled as a % yield, comparing the number at the start with the number at the end of the process, including all waste that occurred during the process.
Use the following equation to determine % Yield =
[No. of Goods produced at the end of process + Rejects + Samples + Returned] ÷ [No. of goods received at the start of process] x 100.
All components and products should be reconciled 100%. However, allowances (Tolerance limits) are made to allow for counting errors and minor inconsistencies.
You must investigate any unusual reconciliation situation, and an out-of-tolerance tally as soon as identified.
In the case of the out-of-range result, you should perform a recount and have the entries corrected.
If the % yield is still outside the allowable limits, a thorough investigation must take place to determine the cause of such a deviation and establish that no mix-up has occurred.
For example, use the following batch reconciliation sheet for a table packing line.
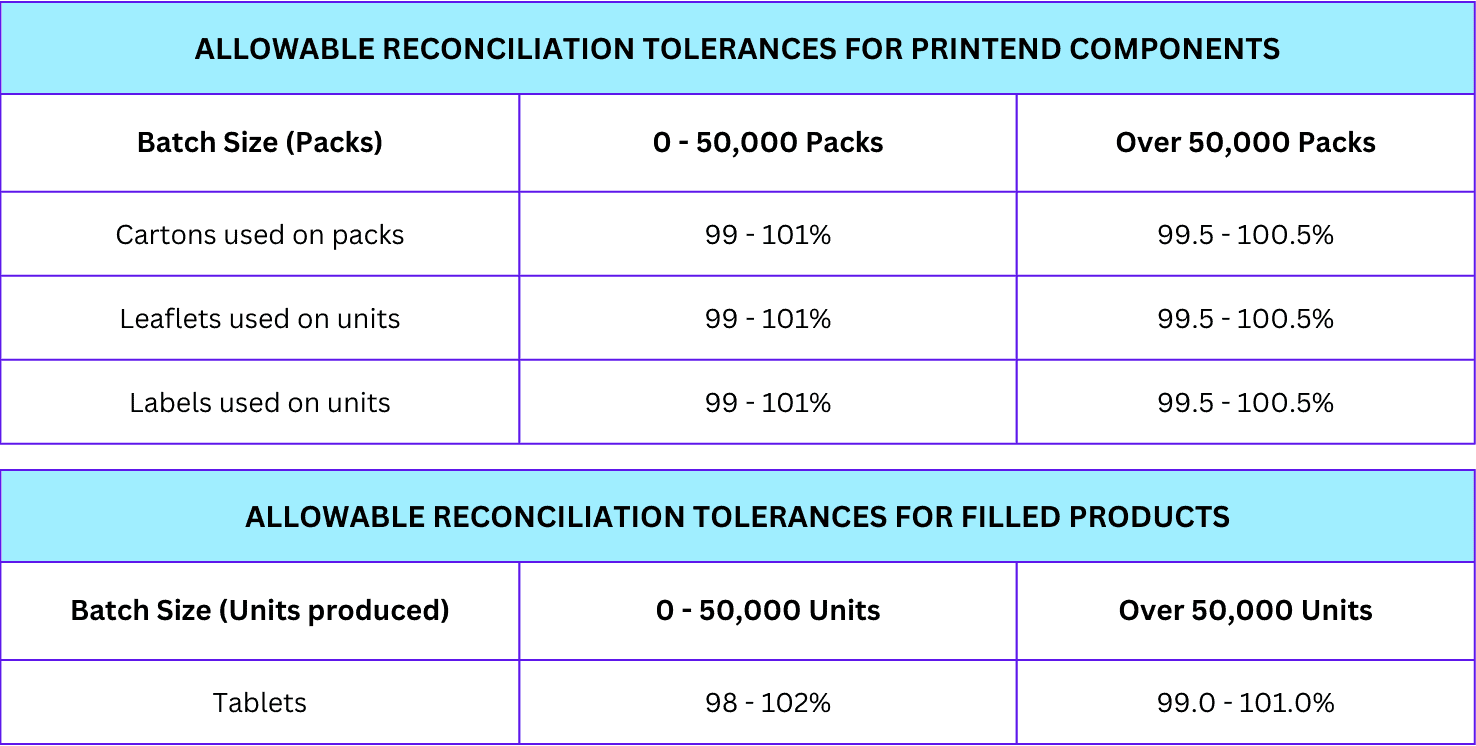
Allowable tolerances for components and filled products
During the reconciliation, you can use the following allowable tolerances for printed components and filled products.
Rules for calculating reconciliation
– Reconciliation calculations must appear on batch records for each batch processed and should be calculated carefully and accurately.
– Estimating or fabricating figures for waste or losses to achieve a 100% result may result in a product recall or, worse still, injury to a customer.
– Companies should use simple formulae and calculation steps to facilitate reconciliation.
– Reconciliation results are only meaningful if they can be compared to acceptable limits or tolerances.
– The limits should be established from a critical review of botch records that reflect good practices.
– The limits may be expressed as the number unaccounted for or as a percentage.
Note: Weighing printed matter is not an alternative to counting. Due to the variability in paper weight, weighing the printed matter is far too inaccurate to be credible.
Some companies use barcode readers and optical verification systems to reduce reliance upon reconciliation.
However, the accuracy and reliability of these devices will need to be defended through validation and regular performance checking.
Reconciliation using numbers
An alternative to using a reconciliation as a percentage is using an actual number of units unaccounted for.
For example:
– 0 in < 5000 units
– 1 in 5,000-7,500 units
– 2 in 7,500-10,000 units
– 3 in > 10,000 units
This is a “tighter” specification because the percentage method allows for increasing numbers of unaccounted-for items when batches are larger.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Conclusion
Line clearance is a pre-operation step that ensures the processing line is free from any leftover products, components, or documentation from previous batches.
It is followed by a line opening, where the correct materials are brought in so the operation can begin smoothly.
line cleaning is performed after operation completion to prevent mix-ups with the next batch by ensuring no component residues are left behind on the processing line.
Successful line clearance, setup, and opening pave the way for commencing the processing line.
Line clearance and component reconciliation are critical in facilitating necessary GMP controls within a packaging and labeling process.
These controls include the management of printed matter movements, such as controlling artworks and printers, limiting access to the label store, and disposing of obsolete and rejected printed materials.
Additionally, line clearance involves validating counting equipment, performing quality control sampling and checking, ensuring adequate line segregation, and continuously monitoring critical process parameters.
These controls encompass material and label identification, counting or measuring the filled container, verifying seal integrity, ensuring the proper functioning of label verifier systems, coding GMP information, assessing pack appearance, and conducting final reconciliation checks.
Through meticulous line clearance procedures, pharmaceutical manufacturers can uphold GMP standards and safeguard product quality and safety.
If you have a wonderful idea or thought on this topic, please write to us using the comment section below.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.


To the gmpsop.com admin, Your posts are always a great source of information.
Thank you so much. Your comment is really inspiring. Please let us know where do we need to improve?