
Quality Assurance Procedures in Good Manufacturing Practices
- Kazi
- Last modified: April 2, 2024
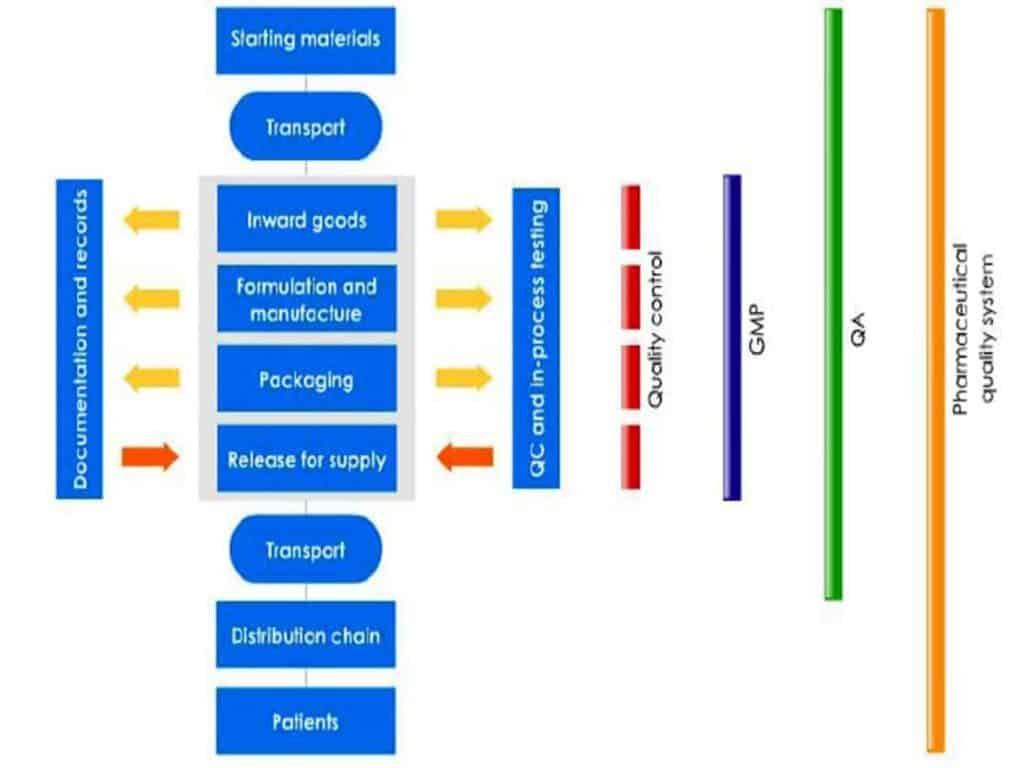
The basic concepts of Quality Assurance (QA), GMP, and Quality Control (QC) are interrelated. The sum total of all these entities together comprises the pharmaceutical quality system (PQS).
The functions or roles of QA, GMP, and QC are collectively critical to the effective and safe production and control of medicinal products.
On completion of this module, you will be able to:
- Recognize how attention to manufacturing a quality product reflects on day-to-day operations.
- Identity the role of Quality Assurance in pharmaceutical manufacturing
- Recognize how companies use GMP rules to minimize errors in manufacturing
- Identify the main roles and responsibilities of Quality Control
- Recognize the key elements of a pharmaceutical quality system
International GMP Rules
Outside the US, almost all regulatory agencies have standardized and harmonized to the EUGMP rules and guidance for medicinal products. Some countries, such as Canada, still maintain a country-specific code of GMP, which is very similar to the EU GMP rules.
In this training program, we use the term “International GMP rules” to refer to the group of rules, guidance, and codes that are based on the EU GMPs. If there are specific differences that are relevant to the module, it will be indicated as such.
Quality Principles
Pharmaceutical products must be manufactured according to well-established GMP rules. These GMP rules have been developed over the last 50 years, and are based on practical experience and accumulated knowledge in the industry.
A quality product refers to much more than one that simply passes its laboratory tests.
US FDA CFR 211
Sec. 210.1 Status of current good manufacturing practice regulations
(b) The failure to comply with any regulation set forth in this part and in parts 211 through 226 of this chapter in the manufacture, processing, packing, or holding of a drug shall render such drug to be adulterated under section 501(a)(2)(B) of the act and such drug, as well as the person who is responsible for the failure to comply, shall be subject to regulatory action.
International GMPs
GMP rules do not specifically define a “quality” product however they do define under what conditions a batch may be released to the marketplace:
“Medicinal products are not sold or supplied before an authorized person has certified that each production batch has been produced and controlled in accordance with the requirements of the marketing authorization and any other regulations relevant to the production, control and release of medicinal products.”
210 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Everybody has a different understanding of the term “quality”. However, in the pharmaceutical industry, quality is very specifically defined. Quality is the sum total of a product’s purity, identity, effectiveness, and of course, its safety.
Purity
Purity means being free of all foreign matter. Contamination to purity can come from incorrect machine setups, as shown here, or by using dirty equipment. An impure product could easily cause dangerous patient side effects, so there is strict GMP for controlling potential product contamination.
Sometimes a contaminant can be present, but cannot be seen. It is very difficult to test for the presence of all possible contaminants.
Identity
Identity means having the right product in the right container with the right labels. If a product is incorrectly identified, a patient may take the wrong strength of medication, or even the wrong medication entirely. These two situations could then easily cause severe health problems, or in extreme cases, death.
Effectiveness
An effective product is one that produces the desired result without any unwanted side effects. Mis-formulating a product or making on error in manufacture could cause a product to lose its effectiveness, leading to patient harm.
Safety
Safety means being free from danger or risks. Consumers have the right to be given drugs that are safe and effective. A safe product does not cause consumers adverse reactions when token at prescribed doses.
Building Quality into Products
Every product must be registered with the appropriate regulatory agency. Products must undergo different levels of evaluation for safety and efficacy, as well as for manufacturing controls. Once a product is approved by the regulatory agency, it may be marketed, provided that each unit in each batch meets agreed standards.
These standards are:
- those standards proposed and agreed to in the regulatory submission; and
- those standards that may appear in official monographs in pharmacopoeias, such as the USP or the EP/BP
Since it is impossible to test every unit in a batch, the only way to assure that products meet standards is to build quality into each step of manufacturing processes. Following GMP rules gives manufacturers confidence in the manufacturing outcomes.
Contaminations
Products are expected to be pure, in that they must not contain any unapproved ingredients or any contaminants. If a product is not pure, it may have an adverse effect on a patient and would therefore become unsafe. An obvious example is bacteria in a sterile product.
GMP rules throughout manufacture of the ingredients and the product help us make a pure product.
Product Labelling
Patients and healthcare professionals rely upon the labelling of a product to ensure that they take the right product and the right strength in the prescribed way.
Product labelling, if wrong, can significantly impact patient health and even cause death. GMP rules, particularly during the packaging steps, help prevent the product from being misidentified.
Factors Affecting Efficacy
During clinical trials, products are verified that they “work”, or are effective. This is confirmed by regulatory agencies when products are approved for commercial manufacture.
Efficacy is largely affected by the amount of active ingredient in the product. For example, 100mg of aspirin is more effective than 50mg in most cases, so it’s important to formulate the exact amount only.
The product excipients (non-active chemicals) can also affect efficacy. For example, some drugs release slowly into the bloodstream due to the right excipients being present. If not, they might release too fast (a safety problem) or too slow (an efficacy/safety problem). GMP rules, particularly around the formulation and manufacturing steps, help to make an effective product.
Building Safety into the Products
Product safety is built into the product during its development cycle during non-clinical and clinical studies. In these phases, products are tested and investigated to see if they have unacceptable adverse effects on patients.
Manufacturers look for a range of “contra-indications” as well as the benefits of the product. During manufacture, it is possible to adversely impact the safety of a drug or biologic by, for example, mis-formulation, adding ingredients in the wrong order, or not following the exact manufacturing instructions. Changing the processing conditions or allowing product to degrade over the shelf life will impact product safety. Applying the GMP rules helps prevent loss of product safety.
Protecting consumers with GMP rules
To ensure that the product has adequate purity, identity, efficacy, and safety in every manufactured unit every time, GMP rules, internal company procedures, and manufacturing instruction must be strictly followed.
Some fundamental GMP rules include:
- following manufacturing instructions, packaging instruction, and registered formulae exactly
- reporting to management any deviation to instructions
- preventing contamination by:
– making sure all process equipment is clean before use
– keeping the facility clean
- preventing product mix-ups by double-checking labels, segregating different products, and conducting line clearances
- completing manufacturing and testing records accurately and thoroughly
Responsibility for quality
Traditionally, GMP has emphasized that the responsibility for quality ultimately rested with the QA and QC groups. However, managers, supervisors, process operators, and support staff all have a role in assuring product quality. Every time a product is handled. Inspected, tested, or processed, the potential exists for quality to be affected. Therefore, GMP regulations require:
- the need for suitably qualified personnel in every aspect of manufacture
- production staff having a role in GMP compliance checking
- QC testing being built into manufacturing processes
Despite quality being everyone’s responsibility, all codes of GMP insist that a separate department (independent of production pressures) oversee and manage the QA systems, and take final responsibility for the quality of batches released to the consumer.
Companies therefore will usually have some combination of a QA department and QC laboratory.
QA Systems
The QA systems:
- implement checks and balances during operations
- review documentation and master batch records
- organize the systems that prevent errors and product failures
- investigate defects and deviations jointly with production
- organize and review auditing and corrective action programs
- release product for sale
Knowledge and GMP behavior
As quality is everyone’s role, personnel are expected to understand how they are expected to behave in a GMP facility. GMP rules recognize that knowledge of personnel behavior toward GMP and product quality is as important as knowledge in GMP principles. As such, personnel need to know what needs to be done, how to complete tasks, and just as importantly, why they need to follow the rules.
To improve knowledge and GMP behavior, all personnel should:
- communicate with supervision and other experienced staff regarding GMP issues, especially if they think a mistake has been made
- if in doubt, ask before acting
- develop knowledge of how the products are used by customers
Quality Knowledge
Knowing how the product is used by patients and clinicians and what effects it may have if incorrectly manufactured provides important understanding for manufacturing personnel. This knowledge enables people to better recognize why certain rules are required, and hopefully recognize when manufacturing deviations may cause patient concerns.
GMP Knowledge
All international GMP rules require formal training in good manufacturing practices across all levels of management and personnel, the reason for this is that the rule have been developed over 50 years, and contain practical experiential-based requirements to prevent manufacturing errors and defective product.
Product/Process Knowledge
Manufacturing personnel should be trained and have knowledge of the products that they produce and the processes that they use, this information is often documented in the master batch or master packaging records. Personnel should be familiar with these before they manufacture any product.
GMP behavior
Minimizing human error in a manufacturing environment is one of the key factors that ensure products are produced in a safe and effective manner. Personnel should always keep in mind that they are making products that are often essential life-saving medicines. The end user relies upon compliant GMP behavior to assure product quality.
GMP compliance program
The GMP compliance program assures the quality of products and protects the consumer. A GMP compliance program is made up off our parts: documentation, training, self -inspection, and corrective action for when things go wrong.
Documentation
Documentation provides personnel with essential information about relevant QA and GMP rules through SOPs, and exact ways to manufacture and test products solely through master instructions and test methods. (Companies may have slightly different names for these documents.)
Companies cannot have a quality plan without proper approved documents in place.
It is a GMP requirement that documentation must be followed exactly. Following documentation also helps minimize mistakes.
Training
Training is an essential requirement under GMP: personnel must be trained before they are approved to perform a job. Untrained personnel will eventually make errors and mistakes that can cost lives.
It is a GMP rule that staffs are trained to the approved documents applicable to their work areas, and they must sign to the effect that they understand the requirements.
Self-inspection (internal audit)
Self-inspections, or internal audits, are requirements under GMP. The purpose of a self-inspection is to independently check that the rules and documents established by QA management are being followed. Self-inspections also provide opportunities to improve procedures and practices in the factory.
Corrective action
Corrective action is required when an internal audit reveals o problem that might affect the quality of a product.
Corrective action may also be required when a problem occurs in production.
Corrective actions must be documented, and incorrect procedures updated, in order to improve the company’s quality plan.
The PDCA Continuous Improvement Cycle
A GMP compliance program follows the same principles for compliance and improvement of quality as the PDCA (Plan-Do-Check-Act) continuous improvement cycle.
The PDCA cycle dictates that a plan for an action should be documented, then personnel should be trained, and the plan implemented (do).
After the action has been implemented, it should be compared (check) against the plan. This is called “internal audit” or “self-inspection” under GMP.
Finally, the cycle requires the company act through corrective action to improve its compliance and performance.
The cycle then begins again by updating the plan to make sure that the corrective action or improvement is standardized, or made permanent.
Quality Assurance Practices
One critical requirement of all drug manufacturers is that products can only be released to the marketplace if they meet all QC specifications, and they have been manufactured in accordance with the details approved by the government regulatory agency, and there have been no unresolved manufacturing errors.
The batch record proides the evidence and the assurance that the product is safe to release. The QA Department is required to independently oversee and review these activities.
US FDA CFR 211
Sec 211.22 Responsibilities of quality control unit.
There shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling, and drug products, and the authority to review production records to assure that no errors have occurred or, if errors have occurred, that they have been fully investigated. The quality control unit shall be responsible for approving or rejecting drug products manufactured, processed, packed, or held under contract by another company.
** Note: The term “Quality Control Unit” may be interpreted to mean a suitable combination of Quality Assurance and Quality Control.
FDA Guidance for Industry Quality Systems Approach to Pharmaceutical CGMP Regulations
This guidance uses the term quality unit (QU) to reflect modem practice while remaining consistent with the CGMP definition in§ 210.3(b)(15). The concept of a quality unit is also consistent with modern quality systems in ensuring that the various operations associated with all systems are appropriately planned, approved, conducted, and monitored.
International GMPs
Chapter 1 Quality Management
1.1. Quality Assurance is a wide-ranging concept, which covers all matters, which individually or collectively influence the quality of a product. It is the sum total of the organized arrangements made with the objective of ensuring that medicinal products are of the quality required for their intended use. Quality Assurance therefore incorporates Good Manufacturing Practice plus other factors outside the scope of this Guide.
QA is a wide-ranging concept that covers all matters affecting product quality. Each company may interpret its QA responsibilities slightly differently.
QA generally includes the oversight and management of:
- compliance with government GMP regulations
- documented procedures and GMP
- control over starting materials
- in-process controls and validation
- QC testing of finished products
- Self-inspections I internal quality audits
- release for sale
- product reviews and trends
- storage and distribution
The need for documentation, records and traceability
GMP regulations require that all stages of manufacture are documented in order to enable traceability. Compliant documentation allows an investigation, if required into the manufacture of the batch. It also allows traceability to where products were sent.
Records that should be retained in a documentation system include records of:
- raw materials
- packaging materials
- manufacturing
- quality control testing
- finished product distributin
- incidents and deviations
- complaints
Documents and Records
Manufacturing documents should clearly define the manufacturing process, be easy to use, and be easy to check.
There are rules as to how GMP-compliant documents and records are handled:
- Standard Operating Procedures (SOPs) and Work Instructions [WIs) must be kept current and must be readily available at the worksite.
- All SOPs, WIs and batch processing instructions must be followed exactly. Any deviations from procedures should be reported to supervisors.
- Batch documents must be verified and approved before issue to manufacturing
It is the responsibility of management to provide a documentation system that conforms to the GMP requirements, and then train the users in the requirements of the system.
Records are the documented history of the manufacturing process. They must be complete and accurate, and demonstrate that authorized procedures were followed.
A key GMP principle is: “If It isn’t documented, It wasn’t done.”
Personnel and training
The manufacture of quality products relies on people. Therefore, it is important that personnel have the right skills, knowledge, and attitudes to perform their work correctly and diligently.
Types of training required by personnel include:
- induction training
- knowledge of GMP rules
- SOP training (understanding of company procedures)On-the-job skills training
The company must be able to demonstrate that training has been conducted satisfactorily in the above areas by maintaining training records for each person.
Human Error and Behavior
The majority of incidents leading to defective drug products result not from failures In technology, but from human error or simple mistakes. The behavior, attitude, care, and knowledge of employees, therefore, are critical elements in manufacturing safe drug products.
The company is required to document procedures and GMP rules, but they have no value unless employees know and follow them in all their work practices.
Internal audits
Well conducted internal audits, managed by QA, help companies identify any weaknesses in any part of manufacturing or testing processes.
Internal audits:
- are required by government
- involve all manufacturing areas
- must report findings and take corrective action
- present opportunities to improve operations and GMP standards
It is important that audits are conducted in a constructive and professional manner and encourage the active participation of the people that are responsible for corrective actions.
Effective audits
The following audits may be conducted:
All audits are designed to ensure the proper procedures and practices are in place and are followed.
During an audit, personnel should:
- Be prepared
- Be positive
- Slick to the facts
- Don’t offer personal opinions
- Don’t offer irrelevant information
External Audits
External audits are generally conducted by purchasers, contractors, and accrediting bodies.
Generally, an external audit will include at least the following areas:
- the quality management system
- written procedures
- validation of processes
Preparing for an audit of QC Laboratory
The laboratory will most likely have to present the laboratory notebooks, test methods, and “objective evidence” on how tests were conducted or results were calculated. It must have all the documentation that might be needed readily available. Certain personnel may be asked to take part In the audit because they are either experts in certain areas, or have particular knowledge of an Issue or event.
Safety
Safety in the laboratory, manufacturing, and the warehouse is critical to a good working environment. All employees must abide by the safety rules, and regular safety audits conducted to ensure that the workplace does not present risks to personnel.
Compliance
GMP rules ·require that personnel adhere to the requirements of GMP and the company SOPs. One way to verify this is for QA representatives to conduct regular GMP compliance audits and oversee corrective action.
Regulatory
Government regulators will visit the factory at least every 2 years on overage to conduct on independent inspection of the facility and manufacturing housekeeping practices. The outcome of the inspection may determine the issue or retention of a GMP manufacturers license, so these audits are very important.
Investigation
Sometimes problems arise in the factory that may raise safety or quality concerns with a product or production line. When this happens, senior management may initiate an investigation or audit of the area concerned to see if the real cause of the problem can be identified and fixed.
Trend Analysis
GMP rules require that products be annually analyzed for trends. The purpose of this is to look for changes in product quality that may be occurring over time. These trend reports must be retained and provided to government auditors if requested.
Corrective Action
A documented action plan should be developed if any major problems arise during on internal audit. The action plan should consider the following, as appropriate:
Activities
List any activities that must be accomplished to either correct the existing problem or eliminate o potential problem.
If there are multiple activities, a corrective and preventive action (CAPA) project plan may be valuable. Assign a person to take responsibility for implementing the plan to the agreed timeframe. If the plan is complex, a CAPA team maybe valuable.
In CAPA activities, it is also important to be realistic about achievable timeframes and milestones.
Documents
Implementing a CAPA usually involves a change to existing practices via the change control program, as well as the updating of procedures, instructions and specifications.
It is important to identify which documents will need to be updated as part of the CAPA plan.
Processes
Either quality system procedures or manufacturing processes are usually revised when implementing CAPA.
If manufacturing processes are to be updated, they may require revalidation as part of implementation.
Once manufacturing processes are changed, it’s important to continue to monitor the effects of the change on product quality in order to provide GMP quality assurance procedure that the change has not had any adverse effects.
Training
Implementing effective CAPAs often requires changes to procedures and stair practices.
When changes of this nature occur, it is a regulatory expectation that supportive training is conducted so that CAPA permanently fixes the problem.
Vendor assurance
Supplier or vendor assurance programs are the first steps in ensuring that quality products are manufactured. It starting materials are sub-standard; the company would have to rely on the laboratory to find any defects through testing. As laboratory testing has some significant limitations, QA is required to implement vendor assurance programs.
QA’s role in vendor assurance includes the following activities:
- Auditing critical suppliers
- Approving or rejecting suppliers whose products may affect qualify
- Changing starting material receipt testing plans, depending on the supplier risk
- Monitoring the supply chain
Change Management
Changes to products and processes are some of the hardest areas to manage in a pharmaceutical company.
Often, changes have to be approved by government agencies before the changes can take place. The QA Department oversees the change programs.
Phase 1
Staffing the process requires a change request to be raised. It is required for the correction of a problem and/or an improvement opportunity. Sources of a change request could be from anyone in manufacturing or QC, or even from regulatory agencies. Change requests are formalized and given unique change tracking numbers.
Phase 2
This phase, overseen by QA, assesses the potential effects of any proposed change (impact assessment). Of prime concern is the potential impact on product quality, reduction, improvement over process controls, and the impact on product registration.
If the proposed change is not allowed by GMP, results in a reduction of product quality, or results in a loss of process control, the change is not implemented. If the proposed change improves or restores process control, or restores product quality, the change management process progresses to Phase 3.
Phase 3
At this change planning phase, there are generally two options for change, minor or major, depending on the change’s impact.
Minor changes are effected by updating documents, and conducting retraining, if necessary. For major changes, a documented change plan is necessary that considers regulatory approvals, validation, training, and documentation. The change plan must be approved by QA.
Phase 4
In this phase the change plan is implemented. The effectiveness of the implementation is verified, usually by QA, and the documentation is finalized. The change is then closed.
Sometimes, QA may schedule a post implementation review to assess ongoing control or improvement.
Release for supply or sale
A formal release for supply or sale is undertaken by QA only after finished product testing has been satisfactorily completed.
Release procedures require QA to:
- review all laboratory tests and batch manufacturing records
- ensure that product is correctly formulated and that any process deviations are explained
- verify that yield and reconciliation calculations are accurate
- verify that labelling and packaging process is error-free
For a batch to be released to the consumer, simply passing all tests is not enough. Quality must be built in al all stages of manufacture.
Reviewing Products for Release
What should a good release for sale review process look for?
- a valid batch number and expiry date
- the batch was formulated exactly as per the master formula
- production is as per the master manufacturing and the finished product meets specifications
- yields and reconciliations are within limits
- the correct labels and printed matter are applied
- there are no unexplained changes, corrections, or alterations in the batch records
- environmental tests or controls are satisfactory
- in-process checks were within expected tolerances
- there were no deviations, or any deviations are fully investigated and explained
The above activities are a combination of checking against specifications (testing in quality) and examination of the process (building in quality). It is not enough to just release product because it passes all tests since only a small proportion of any batch is tested. QA must be assured that all units in the batch would meet requirements if tested and they are safe for the consumer.
After the product is released for sale, the next product review is by the customer or patient. Any problems here will result in customer complaints, or even worse, a product recall.
Good Manufacturing Practice (GMP)
GMP rules have been developed over the last 50 years based on incidents, errors and disasters in drug manufacture. They represent an accumulated body of past experiences written down as rules, so that companies do not repeat past mistakes and put patients at risk.
The GMP rules are there so companies do not learn by trial-and-error. Patients’ lives depend on pharmaceutical manufacturers not making mistakes.
US FDA CFR 211
FDA Guidance for Industry Quality Systems Approach to Pharmaceutical CGMP Regulations
Other CGMP assigned responsibilities of the QU are consistent with modem quality system approaches (§ 211.22):
- Ensuring that controls are implemented and completed satisfactorily during manufacturing operations
- Ensuring that developed procedures and specifications are appropriate and followed, including those used by a firm under contract to the manufacturer
- Approving or rejecting incoming materials, in-process materials, and drug products
- Reviewing production records and Investigating any unexplained discrepancies
International GMPs
Chapter 1 Quality Management – Clause 1.2
1.2 Good Manufacturing Practice is that part of Quality Assurance which ensures that Medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization or product specification.
Good Manufacturing Practice is concerned with both production and quality control. The basic requirements of GMP are that:
- all manufacturing processes are clearly defined, systematically reviewed in the light of experience and shown to be capable of consistently manufacturing medicinal products of the required quality and complying with their specifications;
- critical steps of manufacturing processes and significant changes to the process are validated;
- instructions and procedures are written in an instructional form in clear and unambiguous language, specifically applicable to the facilities provided;
- operators are trained to carry out procedures correctly;
- records … demonstrate that all the steps required by the defined procedures and instructions were in fact taken. Any significant deviations are fully recorded and investigated …
GMP refers to a set or licensing requirements to which companies must adhere in order to obtain and retain a manufacturer’s license. GMP rules have been agreed upon by government and industry, and the rules have been gradually refined during over 50 years or experience in practice.
Internationally, GMP rules are referred to as codes, rules, or guidance to GMP. In the USA, GMP rules (also called current GMP, or cGMP) are legally enforceable regulations. While the USA and international GMP are written differently, there is little difference in practice. The codes are written in a practical way to help manufacturers consistently make quality products.
Applying GMP correctly helps companies minimize errors during manufacture and packaging. GMP, then, always involves people, and the effects or their behavior during manufacture.
GMP requires personnel to document what they intend to do, to do what they intended, record what was actually done, and check the results against agreed specifications.
Scope of GMP rule
Topics covered by the GMP rules include:
- Quality management
- Personnel and training
- Equipment
- Premises and grounds
- Factory sanitation and personal hygiene
- Documentation
- Production control
GMP and documentation
Manufacturing documents and records provide proof of product quality, so it’s very important that a complete set of documentation is maintained.
This means documenting policies and procedures such as:
- manufacturing formulae and instructions
- Specifications
- test methods
All records have to be accurately documented as well, including:
- batch records
- test records
- distribution records
- equipment log books
- training records
Essential GMP requirements
GMP compliance encompasses many activities that are documented in the GMP regulations, codes and rules.
Validation
According to the international GMP definition, validation is “the action of proving (in accordance with GMP) that any procedure, process, equipment, material, activity, or systematically leads to expected results.
The FDA’s definition of validation is “establishing documented evidence which provides a high degree of assurance that a specific process will consistently product a product a meeting its pre-determined specifications and quality attributes.”
Process Control
GMP regulations require that all processes operate “in control”. In practice, this means that production conditions are documented in the master production and master packaging instructions, the instructions are followed by operators, and the process is monitored to ensure that it stays in control.
Batch records provide evidence whether or not the process remained in control.
A deviation occurs when the batch processing is not performed exactly as per the master instructions or SOPs. When a deviation does occur, GMP requires it to be formally documented and reported to QA for review.
All deviations must be resolved before a batch can be released to market.
Contamination Control
A medicinal product is consumed by patients that are unwell or otherwise vulnerable. If the product is contaminated, this may be a health hazard for the patient. GMP rules are largely written to prevent the possibility of contamination of products either by other products, the environment, the facility, personnel, equipment or microbiologically.
GMP rules also place particular emphasis on proper and validated cleaning and sanitation procedures.
Why does GMP require us to validate processes?
One of the essential GMP requirements is to prevent cross-contamination of products by using effective cleaning procedures and processes.
Vessel Cleaning
There are a few ways to check that products being processed in vessel are not cross-contaminated:
1. Sample and test the vessel after each cleaning.
The tank can be sampled and tested every time, but this is expensive, time-consuming, and subject to sampling errors.
Additionally, there is a problem of what to test for: should the tank be tested for detergent residues? Unremoved product? Bacterial contamination?
2. Test the finished product for contaminants.
This approach is not a reasonable or economic approach. Again, what possible contaminants should be tested for? What will be the cost of rejecting the batch if a contaminant is found? What would be the health risk to the patient if the contaminant wasn’t detected?
3. Validate the cleaning process.
Validating the cleaning process would involve demonstrating under a set of specific conditions (the approved cleaning procedure) that all contaminants can consistently be removed. If the conditions can be reproduced usually three times, the cleaning process can be validated.
When the process is validated, then the company no longer has to rely on intensive and expensive testing to prove cleanliness each lime.
What needs to be validated?
There are many things that require validation under GMP. These include:
- the suitability of the manufacturing facility
- the suitability of critical utilities such as gases and water
- GMP-related computer systems
- production equipment
- equipment and facility cleaning procedures
- laboratory fest methods
- manufacturing and packaging processes
Test Method Validation
The laboratory is required to demonstrate that all test methods are reliable. This means they must be validated by verifying the following attributes:
- precision (amount of variation in the assay)
- accuracy (difference between the average results and the “true” value)
- selectivity (ability of the method to measure the analyte in the presence of Interfering compounds)
- sensitivity (limit of quantitation or limit of detection of the method)
- linearity and range (over what range the method measures the analyte in direct proportion)
- ruggedness (how the test method is affected by reasonable changes)
GMP regulations require that all critical steps of manufacture are reliable or validated. If a method is not validated, it is difficult to be assured results are reliable.
Process Validation
Over the last years, the industry has learned by experience that:
“Quality cannot be tested into product, but must be built in at each stage of manufacture.”
This statement is the basis of process validation. Process validation assures that under specific and documented operating conditions, a process step will perform reliably, be in control, and will deliver a quality product every time. Limited laboratory testing is conducted to confirm that the process is still operating satisfactorily. To conduct process validation, the following must be defined:
(a) Process inputs: The critical process parameters, or CPPs, that may affect process reliability. Often, CPPs could be time, temperature, speed, order of addition, etc.
(b) Process outputs: The product critical quality attributes, or CQAs, that the process delivers. Examples of CQAs include strength or potency, particle size, moisture, purity, weight, appearance, and dissolution. CQAs will vary with the type of product manufactured.
GMP and Process Control
Under GMP, all manufacturing process steps must be “under control”. After processes are initially validated, to maintain control:
- Inputs (CPPs, or critical processing parameters) should be monitored throughout the process.
- Outputs (CQAs, or critical quality attributes) should also be monitored against tolerances.
Adjustment to process steps are also part of maintaining control. Most manufacturing processes have documented tolerances or control limits, which if exceeded, require adjustment of the process, and sometimes raising a deviation report. Processes require adjustment if they drill into the upper and lower action zones as shown in the diagram.
Process Deviations
Rejects and recalls are often associated with significant deviations from standard processing conditions, so deviations should be assessed before botch release. GMP requires that deviations should be properly documented when they occur in order for QA to investigate them.
Some of the QA/GMP requirements for managing deviations include:
- QA should review process deviations.
- Planned batch deviations must be approved by QA.
- Deviations during production must be recorded.
- Batches must not be released until investigations are complete.
- Reprocessing or reworking must be approved by QA.
Contamination Control
One of the fundamental aims of GMP is to provide rules to prevent product contamination from occurring, as contamination is almost impossible to detect once it happens. Since many patients are already unwell, contamination may make them more unwell.
Not all contaminants can be seen (e.g. bacterial contaminants), and so strict controls are needed to prevent contamination. Personnel should record on the batch records if they suspect contamination has occurred, as well as report it to supervision.
GMP Rules and Cleaning
The GMP rules are very explicit regarding the requirement to clean and sanitize equipment and the manufacturing facility.
Since most residues cannot be seen nor reliably tested tor, the company’s standard procedures and cleaning records are relied upon as evidence of cleaning.
Cleaning Procedure:
GMP requires the cleaning procedures to be fully documented in written procedures. These procedures are initially validated (shown to be effective) specifically for an item of equipment or on area.
Once validated, the procedures are then published and used.
Cleaning Validation:
All cleaning procedures should be validated (shown to be effective) under specific conditions of use. These conditions of use ore specified in the written procedure.
If employees alter the conditions of use, the cleaning methods may be “invalidated” and may not be effective, for example, it would seem logical that adding more sanitizer than the procedure requires would make cleaning faster or better. This is not always the case. Adding additional sanitizer may alter the pH of the cleaning agent and make it less effective.
The relevant GMP rules are to only clean under validated conditions, and to follow procedures exactly.
Cleaning Conditions:
The written procedures describe the required conditions under which cleaning is optimal. These conditions may include a range of the following:
The written procedures describe the required conditions under which cleaning is optimal. These conditions may include a range of the following:
- strength of the cleaning or sanitizing agent (e.g. 2.0% v/v)
- the type of water or solvent to be used
- the pH of the cleaning agent
- the temperature of the water to be used
- how much to dismantle equipment before cleaning commences
- the contact or residence time for the sanitizing agent on the surface
- what agents to use to clean off the sanitizing agent
- what standard of water to use in the final rinse (GMP, for example, requires purified or water for injection as the final rinse)
Cleaning Records:
One essential GMP rule is the keeping of detailed cleaning records. Cleaning records provide not only proof that cleaning took place, but also provide evidence of the cleaning outcomes, for example, that the surfaces are visually clean, or the results of rinse water tests. The records must also identify who did the cleaning and when, and must be signed.
For automatic cleaning procedures such as clean in place (CIP), the cycle conditions are usually automatically monitored and the conditions recorded. Cycle conditions may include the temperature, flow rate, time, concentration of solvent, and solvent agitation time. Often CIP systems have alarms when something goes wrong.
The completed and signed records should be attached to the records, since they provide the only real evidence that cleaning has occurred.
Conclusion
Quality is everyone’s job, and it should pervade every aspect of pharmaceutical manufacture and packaging. Quality should not be tested into products, rather, it must be built in at each step or manufacture.
Quality manifests itself in not only obvious ways, such as during actual processing steps, but also in diverse areas such as in vendor assurance, personnel training, internal audits, change management, release for supply, and QC sampling.
Pharmaceutical manufacturers must integrate the key functions of quality control (which checks for defects after they have occurred) and regulations or GMP under the banner of quality assurance. QA is charged with preventing defects and errors from occurring by overseeing all aspects of producing a quality product.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
