Finished Goods Return Procedure in GMP
- Published on: Oct 16, 2018
Typically, medicinal products are manufactured, tested, released and distributed under highly regulated environment commonly known as cGMP (Current code of Good Manufacturing Practice) strictly enforced by local Regulatory Authorities.
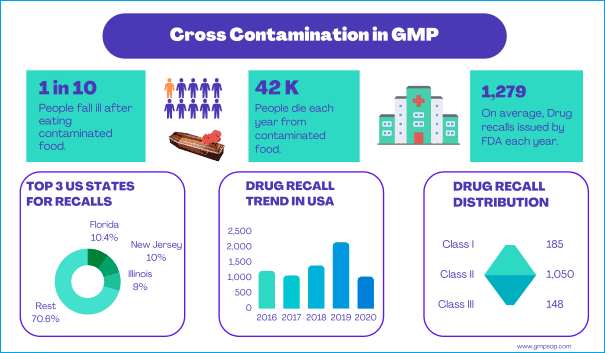
Even though medicinal products have to comply such stringent manufacturing guidelines and right first time activities, some released medicinal goods have to come back to their sources due to general customer dissatisfaction, complaints of packaging damage, physical abnormalities, evidence of adverse reactions or lack of efficacy etc. Sometimes company initiates voluntary recalls as a precautionary measure. Hence, each GMP site should have well established standard procedure to describe the process and responsibilities for receiving, evaluating, repackaging and/or cleaning and disposing of returned finished goods.
All finished goods returned from the market would undergo four handling steps below:
- Goods receipt and storage
- Product evaluation and disposition
- Product repackaging and / or cleaning
- Product disposal
How to Manage Returned Goods:
Briefly, the company Product Manager approves the return of goods, a credit and/or replacement of stock. QA evaluates and provides a disposition on all finished goods returns. Warehouse store personnel handles and dispose the returned goods in accordance with environmental and / or appropriate standard operating procedure. Company distribution team ensures all finished goods that are assigned as ‘Reject’ are properly disposed off. The Production team ensures the goods with “Rework’ tag are processed as per approved rework procedures pending further QA release.
Role of customer Service:
Typically, customer contacts the Customer Service or Product Manager to request return of goods. The Customer Service team in advice from Product Manager organizes the return of stock from the customer, conduct the triage investigation the reason for return and issue a credit note or replacement product (if required). Customer Service will arrange for product return as sales returns and will contact a freight company to have the goods picked up from the customer and delivered to the warehouse. Stock requiring storage between 2° and 8°c will require temperature monitoring.
Role of Warehouse Store:
Warehouse receipts the goods as sales returns, book into inventory system as quarantine stock ensuring controlled storage of all returned goods in the secure storage ‘RETURN’ location pending disposition by QA.
As the goods arrive the Warehouse employee will receipt the returned stock and apply ‘Under Inspection’ stickers to the product and transfer goods to the secure storage ‘RETURN’ location in the warehouse pending an inspection and disposition decision by QA.
Stock requiring storage between 2° and 8°C is stored in the temperature controlled cool room. Warehouse initiates a “Returned Goods” form (Customer Return and Returns Confirmation) and forward to QA for a product inspection and disposition decision.
Product Evaluation and Disposition by QA:
QA will review returned goods on a weekly basis unless otherwise requested. QA staff will critically assess the returned goods using the following assessment considerations:
- The nature of the product (e.g. perishable, non perishable, sterile, non sterile)
- Special storage conditions (e.g. 2 to 8°C)
- Condition and history of product (e.g. no evidence of tampering, leaking, damage or temperature excursions).
- Time elapsed since the product was issued to the customer. If the time elapsed from issue to customer to return date is greater than 2 calendar months then justification for acceptance of return should be provided by the Customer Service department.
- Remaining shelf life on stock (is stock short dated?).
- Returned goods requiring cold storage will be dispositioned as ‘REJECT’ if supporting data for product storage at 2°-8°C is not available.
- Complete rest of the QA section of “Returned Goods” Form and assign disposition as either: Return to stock, Rework or Reject
- Update the ‘Returned Goods Log’ or electronic inventory (MRP) system and complete the “Returned Goods” form by assigning a Goods Return Number.
Based on disposition decision, the QA will assign a status to the returned goods in the MRP system. QA will notify the Warehouse of the stock disposition. If disposition decision is REJECT, product will be rejected with appropriate procedure. If disposition decision is REWORK, product will be reworked by Production Operation team by assigning a REWORK batch number (typically with a suffix of letter ‘R’ after the original batch number) but keeping the original expiry date.
In case of third party manufacturer products, returned goods that require to be reworked, are returned to the site of manufacture.
As an exception when replacement of non registered packaging only (e.g. shipper) or cleaning of external packaging is required, repackaging and / or cleaning can be performed by warehouse personnel and record appropriately (may be using Returned Goods form). A rework procedure will not be applied.
Final product disposition will be assigned by QA on completion of rework, repack and / or cleaning. Based on disposition decision, the QA will assign a status to the goods in the MRP system and / or label the goods in a way to clearly indicate the status of the goods.
The completed “Returned Goods” form and any other related paperwork such as print out from MRP system, customer correspondence, warehouse cleaning records etc. are filed together in the ‘Return Goods’ folder and stored in QA office.
Materials Management of Stock:
- Return to Stock:
Distribution team will transfer the stock being dispositioned by QA as return to stock from Location RETURN to an identifiable stock location.
- Reworked Stock:
Distribution team will notify the Planner of required Rework so the reprocessing and/or repackaging can be placed into the production schedule and documentation can be issued. Stock will remain in the non-allocatable RETURN location until issued for rework.
In case of non registered returned goods Distribution team will schedule and record repack of non registered packaging and / or cleaning of external packaging and record the work in the “Returned Goods” form. Stock will remain in the non-allocatable RETURN location until inspected and a final disposition assigned by QA.
- Rejected Stock:
Upon notification of stock dispositioned as Reject by QA, the Distribution team will seek approval for disposal. Stores personnel must dispose of goods in accordance with environmental procedures approved by the Safety, Health and Environment regulations

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
Basic Overview of Contamination Control in GMP Facility
Data entry on quality control laboratory records
Line clearance procedure and reconciliation in GMP