Warehouse material handling for pharmaceutical industry
- Kazi
- Last modified: March 13, 2025
Warehouse plays a significant role in ensuring pharmaceutical product quality. Warehouse material handling activities are at the heart of providing such high-level assurance.
Mistakes in these activities can significantly affect patient safety or cause batches to be rejected if discovered early.
Therefore, the utmost care must be taken during material handling, such as receiving raw materials, logging in, storing, sending them for testing, and issuing approved raw materials.
Many mis-formulation incidents commence with the wrong issue of starting materials or other chemicals from the store.
The GMP rules clearly state that each issue of starting materials from the store must be accompanied by requisition paperwork and that cross-checks are conducted to ensure that the issued materials match the requisition.
This requirement is based on the fact that material issues are the first point of control inside the factory. It is much better to prevent incorrect chemicals from being issued in the first place rather than find them (or not) later.
Mis-formulation can cause patient medication to be unsafe, which can result in serious injury or even death of patients.
Warehouse material handling is a complex process that extends beyond the mere movement of starting materials.
It necessitates precision, efficiency, and above all, strict adherence to best practices.
This includes managing the storage of raw materials and finished products, maintaining temperature and humidity control, ensuring the proper release and dispatch of finished products, and effectively managing customer returns, counterfeits, and product recalls.
This article is crafted with real-life experiences and insights to help you fine-tune your material handling processes, optimize efficiency, and ensure compliance while working at the warehouse.
Table of Contents
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
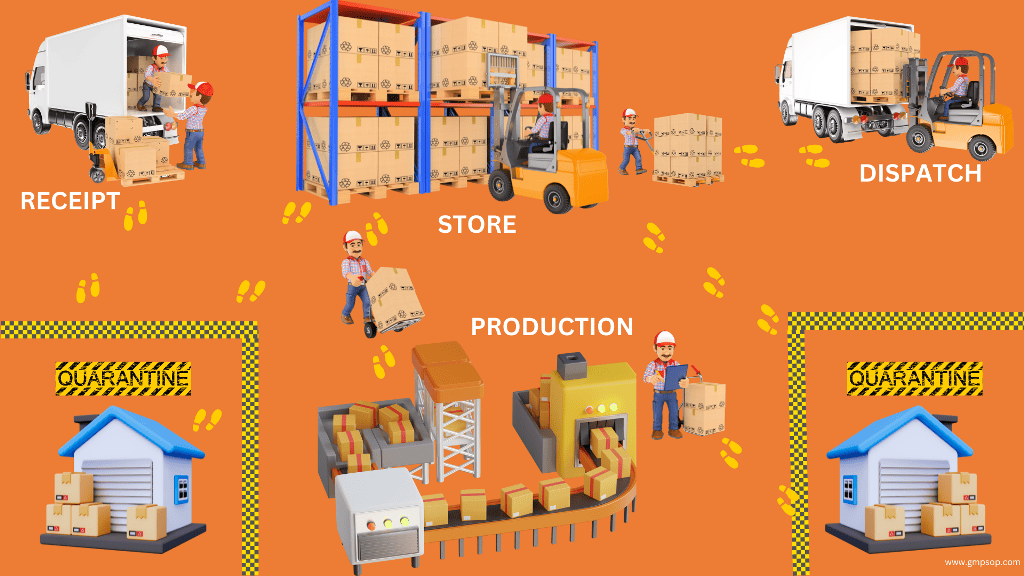
Warehouse material handling of incoming materials
One of the most important aspects of GMP is ensuring that the right raw materials are received and tested for expected quality. Then, the correct raw material batch is issued to production to commence manufacture.
To ensure compliance with GMP requirements, follow the steps below when receiving incoming raw materials.
i. Warehouse inspection at the material receipt
Warehouse material handling starts with the receipt of incoming materials.
GMP commences when raw materials from the supplier are received. Raw materials must be inspected to confirm that the containers are intact, have been provided according to the paperwork, and have labels that identify the raw material name, batch number, and expiry date.
When the raw materials, components, and packaging materials arrive from the supplier, the first thing you should do is verify that the goods match records such as the purchase order and delivery docket.
You must inspect the quantity, supplier’s labels, and container to ensure the incoming materials are:
– Free from apparent physical damage and contamination.
– The material can be identified correctly on the label, and
– The correct quantity is according to the purchase order.
When bulk raw materials arrive in bulk carriers (e.g., tank trucks or railroad tank cars) you should check those are not pressurized.
The tamper-evident seals are in place and intact on the openings and capped discharge lines.
Actively check for tampered container seals, missing containers, signs of physical damage, or evidence of contamination, which are unfortunately common occurrences observed during the receipt of incoming materials in bags, drums, casks, or any other container immediately after unloading from the truck.
Some bulk carriers (e.g., non-dedicated tankers) may also be used to transport different bulk materials.
In such a case, you must verify the evidence of cleaning (e.g., cleaning certificate) before you should accept the bulk materials.
You should not accept incoming materials that do not meet the above requirements and notify your quality team immediately about the unacceptable material.
ii. Store incoming material in quarantine cage
After the first level of successful inspections, the material was stored under quarantine. While the material remains under quarantine the qualified personnel can commence sampling activities.
A quarantine cage should be treated as a prison cell, except the facility is for storing freshly arrived raw materials.
A raw material quarantine cage is part of the warehouse. You’ll need to lock the facility except when authorized personnel complete their activities.
Materials that will have direct product contact during production should be treated in the same manner as incoming raw materials. Some examples of these materials are product filters, filter aids, cleaning agents, lubricants, etc.
When raw materials are stored, they may deteriorate if exposed to elevated temperatures and moisture.
To protect temperature-sensitive materials from deterioration, store them in the designated temperature and moisture-controlled zones per the product’s requirements.
Never store materials directly on the warehouse floor or touching the walls.
Only in rare circumstances will quarantined materials be used for production. For example, urgent delivery requirements of out-of-stock orders.
If this is to happen, you have to initiate a planned deviation and conduct a risk assessment.
Be careful to quarantine the entire manufactured lots in case laboratory testing fails on either the raw material samples or the finished product samples.
You must implement very strict controls in place for those unusual circumstances.
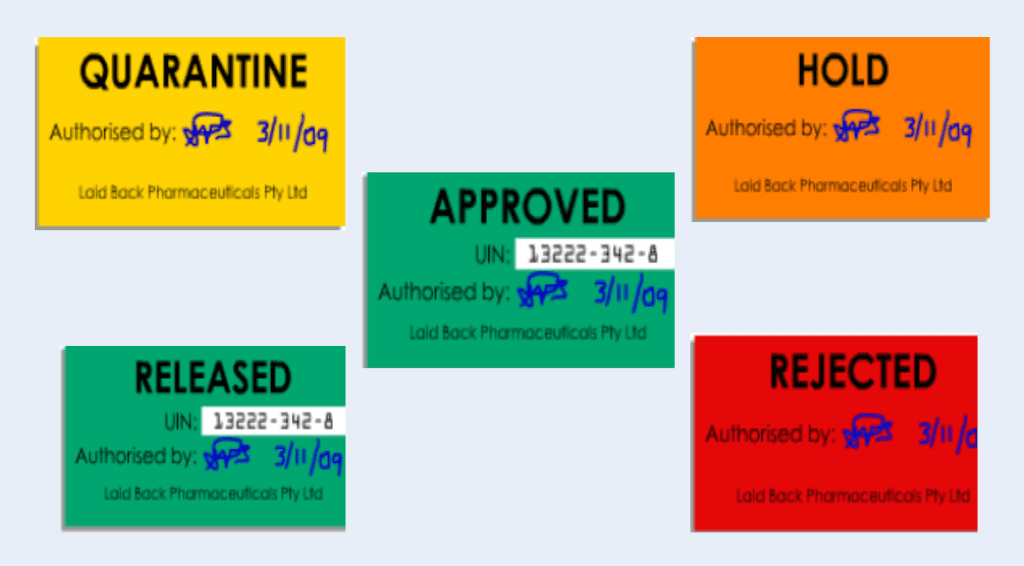
iii. Warehouse assigns status labels on the incoming materials
On initial receipt, you should assign status labels on the raw material containers that should indicate either “Hold” or “Quarantine,” and once they have passed quality control testing, raw materials should be re-labeled as “Released” or “Rejected.”
The storage location must match the status of the raw material. For example, materials with Hold, Quarantine, or Rejected labels must be kept in a quarantine location.
According to FDA Guidance for Industry, Q7A, you must only use “Released” raw materials in production.
Assign status labels to facilitate in-house control while the materials are in quarantine before initiating sampling. The status label should display the following information:
– The name of the material is the same as the specification.
– The in-house material code, and
– The in-house lot number.
– Current status of the material (i.e., quarantine, under test, released, etc.)
Assigning in-house lot numbers for every container received next to the supplier’s lots of raw materials or packaging materials is important.
iv. Complete warehouse records of incoming material
Warehouse staff must complete records for the receipt of materials for each shipment of each lot.
The record should include information such as:
– Copy of purchase order.
– Packing slip or delivery note.
– The identity of the material is by its name in the specifications.
– Total quantity and number of containers received.
– Supplier name and supplier lot number, (if available).
– Name and location of the manufacturer (if known).
– An in-house lot number is assigned to each shipment of each lot (to be used for recording the shipment’s disposition, use, and reconciliation).
– Date of receipt and the name of personnel who received the material and
– Relevant comments made by personnel during receipt.
v. How do you manage multiple lots delivered on the same pallet?
Sometimes, different lots of the same material or even different materials arrive on the same supplier’s pallet. The outer containers often look alike, which can lead to confusion. This is why your vigilance and attention to detail are so important.
Always double-check each container and physically separate it from other lots, even of the same material, on receipt. Do not mix up different lots, as this problem is unlikely to be picked up later in production.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Incoming printed material handling in the Warehouse

The pharmaceutical industry’s printed packaging materials are critical to the warehouse’s management as they contain important information such as product name, ingredients, dosage, expiry dates, batch number, manufacturer’s details, etc.
In case you don’t know, here are some examples of printed packaging materials:
– Product labels
– Cartons
– Carton inserts
– Leaflets
– Blister foil
– Barcodes
You must control the movement of printed materials compared to the rest of the packaging materials as they contain GMP critical information in compliance with your regulatory authority’s requirements.
It is a must to have the printed information clear, legible, and resistant to fading or ink smudging during storage, handling, and transportation.
Follow the steps below to handle printed packaging materials.
i. Dealing with incoming printed materials
When printed materials arrive at the warehouse, you should apply the same principles of material handling as those for incoming raw materials.
Direct control over product quality begins when printed materials arrive at the receiving bay.
If you receive a large number of printed materials for various products at the same time, be very particular in accepting them according to the warehouse’s standard operating procedure.
This is a time when there is a high risk of possible mix-ups.
Train your staff adequately to manage the pressure. Warehouse staff must ensure that the right printed material is received, which will eventually be released by quality control and used by the packaging lines.
When printed materials arrive from the printers, they must be given a unique identification number (UIN) and then placed into quarantine.
Since many labels, cartons, and leaflets look alike, quality control personnel must take particular care in sampling and testing.
You should never allow any packaging and labeling samples to be returned to inventory.
Only after printed materials have passed inspection, they should be released for further use.
Upon the printed materials arrive, you should count the rolls of labels and other printed items before they can be released for the packaging line.
Do not accept counts or numbers provided by the supplier unless the supplier is specifically qualified and will certify the exact count for each roll.
Checking the supplier’s label numbering is an acceptable alternative. Cut labels must be counted and effectively verified by the manufacturer because of the risk of mix-up.
ii. Store printed material in a quarantine cage
Printed material must be quarantined from use until it has been thoroughly inspected by quality control.
Quarantining involves placing the received material in a locked store that is separated from the main warehouse and secure from unauthorized access.
When handling printed material, all personnel should double-check that the “Quarantine” label has been replaced by an “Approved for use” label.
If it hasn’t, do not use the printed material.
iii. Sampling plans for printed material
Quality control uses sampling plans to specify the number of samples of printed material to be taken.
Quality control can use several types of sampling plans, e.g., statistical, targeted, and zero acceptance numbers. Although quality control uses all of the sampling plans for printed material at different times.
To learn more about the sampling plan and acceptable quality limit, read this article.
Sampling plans for printed material should account for at least the following:
– The quantity of printed material received.
– The quality of printed material required.
– The nature of the material (e.g., primary and printed materials)
– The production methods
– An audit of the printed material supplier
– A developed classification of defects and AQL%
iv. Quality control inspection of printed material
Newly arrived printed material must undergo quality control inspection in the same way as starting materials.
You should develop specific sampling plans for different printed materials, such as labels, leaflets, inserts, cartons, etc., that contain product-related information.
Following receipt and quarantine, printed material is examined and verified against quality control protocol, including matching with standard specimens (pre-sample) and specifications, to avoid any mix-ups or errors.
Quality control should compare the sample of printed material lot directly with the official and approved standards or specimens.
Quality control staff should be aware of any latest changes to the materials so they can replace the standard sample and specification to reflect the latest version.
During the inspection, printed material lots must be stored in quarantine and not issued for production use.
Always ensure that the printed material lots are released by the inspection team and that the status label is “Approved” before it can be used.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Warehouse verification of correct material
While laboratory personnel conducts the sampling and testing to confirm that the raw material received meets the quality control specifications, the warehouse also plays its part in ensuring correct materials are received.
i. Warehouse to verify approved suppliers
GMP requires you to maintain an approved supplier list, and starting materials may only be purchased from supplies on this list.
When the shipment and paperwork arrive on the delivery truck, the receiving person should check that the supplier is on the approved list.
Shipment from non-approved suppliers must be set aside and notified to the Supervisor immediately.
Some of the factors that contribute to a supplier being approved are:
– Completion of a supplier questionnaire
– Successful purchasing and QA supplier audits
– Laboratory verification of quality by testing samples
– Provision of an authentic Certificate of Analysis (C of A) verified by the laboratory.
– On-time delivery
For a supplier to remain on the approved supplier list, they must have a good history of acceptable deliveries (e.g., 3-5 consecutive approved consignments).
The purchasing department usually only purchases from QA-approved suppliers, but occasionally there may be a mix-up.
Always double-check the supplier origin to make sure that the supplier is on the approved supplier list before accepting the delivery.
ii. Warehouse to verify chemical name
Many raw materials have similar and sometimes confusing names.
For example, “Ergotamine” can be confused with “Ergometrine”, which are two very different drugs.
Similarly, the same item can be called by different names by different suppliers, for example, “sodium chloride” can be called “sodium salt”.
Pay particular attention to the names of the material being delivered. Sometimes a supplier can mix up a delivery and send the wrong raw material with the right purchase order.
Always double-check the name of the product needed against the paperwork of the supplier.
iii. Warehouse to check standard names register
GMP rules requires you to have standard names register and assign the material name with a unique standard company item code.
The purpose of this is to ensure that everyone in production uses the same terminology to avoid confusion. These names and codes should match the master bill of materials.
When labeling starting materials, always use the company standard name and matching code.
iv. Warehouse to verify the grade of the material
The purchasing department only purchases QA-approved grades of chemicals, such as USP, BP, or EP grades. The grade should be printed on the supplier label next to the name.
Double-check the grade against the purchase order and supplier paperwork before accepting the lot.
v. Verify Material Data Safety Sheet (MSDS)
An MSDS is provided by the supplier and is designed to provide both workers and emergency personnel with the proper procedures for handling or working with a particular substance.
MSDSs include relevant information for the GMP warehouse. Such as:
– Health effects and first aid
– Storage
– Disposal
– Spill/leak procedures
The MSDS is meant for:
– Employees who may be exposed to hazards or who need to know the proper methods of storage
– Emergency responders (firefighters, hazardous material crews, emergency medical technicians, emergency room personnel)
– For safety responders, therefore, it is important for warehouse staff to be cognizant of the materials coming into and going out of the warehouse.
Issue of materials to production by warehouse
The issuing of raw materials means giving away part of the approved or released materials for production use.
As a warehouse member, your role in handling different materials is crucial. You are entrusted with the responsibility to issue raw materials only against approved requisitions provided by the production planning team, ensuring the smooth flow of our operations.
GMP requires that all raw material issues are accompanied by the correct paperwork so there is no potential for mix-up during material handling.
It’s important to understand that the incorrect selection of raw materials can lead to significant problems during manufacture. If not rectified on time, this could potentially cause serious harm to patients. Your vigilance in this matter is paramount.
Only designated and trained staff may issue approved materials. Other staff should never “help themselves.”
If an incorrect material is issued, a disaster such as a mix-up or product failure may follow.
Here are some critical GMP rules regarding the issue of raw materials:
According to GMP rules, Materials should only be issued from the store by authorized personnel following a specific written procedure. This ensures the process is standardized and all necessary checks are in place.
– Records of the quantities receipted into stock and the quantities issued to manufacturing must be maintained.
– Inventory records should be maintained so that any discrepancy between the quantity received and the quantity issued can be seen.
– Discrepancies are to be reported and investigated.
It should also be noted that when transferring goods from the warehouse to production, all goods must be removed from wooden pallets and placed on plastic pallets.
Computer control of released warehouse materials
Rest assured, computer systems are in place to control the inspection and test status of materials and products. This means that materials and products may not need physical status labels or even be stored in separate areas if computers are used, streamlining our operations and ensuring accuracy.
GMP allows a computer system to control the status and will enable the warehouse to release approved materials to the production team.
If computer systems such as Enterprise Resource and Planning (ERP) are in use, they must be validated and secured. Please make sure that you know your computer system procedures and security controls.
Only quality control-nominated personnel can change a material status from “Quarantine” to “Released” after full-length testing. The computer system facilitates that control.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Warehouse storage of raw materials & finished products
Safe and orderly storage of raw materials and finished products is one of the critical activities the warehouse has to perform as part of material handling responsibilities.
You must plan the storage and movement of materials to help production processes achieve efficiency, reduce waste, maximize space, and guarantee the safety of your personnel.
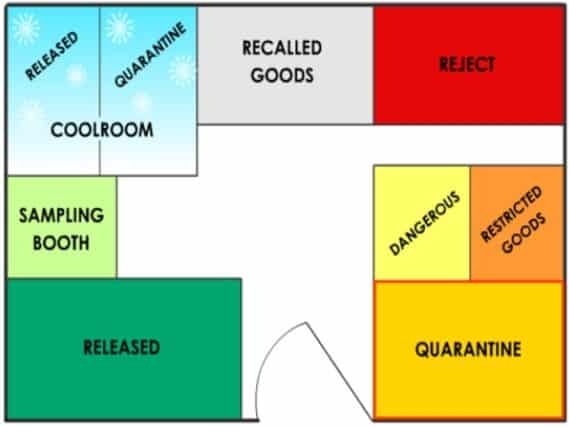
A well-organized warehouse layout is not just beneficial. It’s a necessity for our operations. It plays a vital role in ensuring safe and efficient material handling.
Map the temperature and humidity profiles of different parts of warehouse storage facilities.
Where required, store materials and finished products only in prescribed storage conditions and facilities where temperature and humidity are controlled.
i. GMP storage requirements for warehouse
You’ll need to store starting materials and components in designated locations.
Some materials are required to have additional safety and security procedures.
For example, the following goods must be locked up or otherwise secured from unauthorized access:
– Printed packaging materials
– Drugs of addiction, antibiotics, and highly potent drugs
– Poisons and flammable goods
– Rejected and recalled materials
– Returned goods
Keep powders, liquids, and corrosive and flammable materials separate.
Dangerous goods areas should be specially equipped to prevent contamination and spills.
ii. Temperature monitoring of stored materials
Monitor the temperature of the warehouse storage facilities to maintain product integrity throughout its shelf life.
Temperature-sensitive materials can easily deteriorate by prolonged exposure to temperature.
If it remains undetected, this can cause serious harm to patients as their therapeutic function can be reduced greatly. The worst part is that it could not even be apparent.
You should have a plan for re-testing starting materials after long storage or exposure to adverse conditions. Stored starting materials may not be used for extended periods.
If stored correctly under controlled temperature conditions, they will be good until the expiry date.
Where necessary, validate the effectiveness of the temperature and humidity control system as proof of the efficacy of material shelf life.
Sometimes, the laboratory wants to retest materials before continuing to use them. This is usually a precaution for materials that could, for example, pick up moisture during storage.
iii. Warehouse stock rotation
GMP rules require that starting materials, printed material, and primary packaging be rotated so that materials that may be closer to shelf life or degrading are not left in the store for use.
You are required to practice a “FIFO” policy. FIFO (first-in, first-out) traditionally means the first stock that arrives is the first stock picked for use.
In practice, many companies use “FEFO” (first expiry, first out), implying that the materials with the shortest expiry must be used first.
The FIFO principles also apply to the finished product. Customers often reject stock with a short expiry date.
Warehouse staff should know this policy and follow the company’s written procedures.
When selecting stock, always check its expiry date to ensure FIFO principles are adhered to.
If you have any doubts, please feel free to refer to management.
iv. Rejecting defective and expired materials from the store
Starting materials and packaging materials may not meet quality standards on receipt or may reach their use-by date (“time expire”).
It is common for starting materials and finished products to reach their use-by date while in storage.
Many companies have specific rules about only releasing finished products that have, for example, over 50% of their shelf life remaining.
This is because there are risks that customers may reject the finished products due to their very short shelf life.
Companies also have rules regarding the receipt of “short-dated” starting materials.
Management should be alerted if incoming goods have, for example, less than 75% of their expiry period left.
GMP rules require that the expired stock be securely disposed of so that there is no chance it finds its way back into manufacturing or onto the market.
You should develop specific secure disposal procedures to control this activity.
GMP rules require that you have written procedures for handling and rejecting defective or expired materials. (Reference: Regulatory Considerations for Raw Material Qualification and Related Controls for Biotechnology Drugs).
On rare occasions, subject to strict QA approvals, permission may be given to extend the expiry of starting materials, provided they meet retesting by the laboratory.
v. Finished product release
Pharmaceutical customers expect to receive a product that is not only manufactured and packaged correctly but also arrives in good condition.
Warehousing, transportation, and distribution procedures are vital in protecting the product to the customer.
i. Release to Quarantine
After manufacturing, the packaging department checks that all the batch documentation is complete and accurate and the product has been correctly packaged and labeled.
The batch is then assigned to the Quarantine area in the warehouse.
ii. QA clearance
GMP rules require that quality assurance personnel conduct a final audit or clearance of the manufacturing and packaging records.
This is to independently verify that the product has been correctly manufactured, packaged, and tested.
If a batch has any questions or unresolved issues, QA will hold up its release until these remaining issues are closed out.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
i. Preparation for dispatch
The warehouse team, as a crucial part of our operations, will receive a picking or dispatch order for either the whole batch or for smaller portions of the batch. Your role in maintaining the quality of our products is paramount.
At this critical stage, it is your diligent cross-checking of the order against the batch details that can prevent the dispatch of a finished batch with quarantine status. If such a situation arises, please raise the issue immediately with management.
On rare occasions, the quality team considers whether to allow finished products with quarantine status to be transported out of the warehouse. Typically, this process is called “Release under quarantine.”
A planned deviation is raised and approved for this purpose. This is the company’s risk of rejecting the whole order if the laboratory testing fails.
Rest assured, before shipment, the order must go through a series of protective measures to guard against possible transport damage or deterioration. These include the addition of outer packaging, shrink wrap of pallets, and labeling of transport temperature conditions.
ii. Special precautions to take during dispatch
Things can still go wrong in the warehouse even after the batch has been successfully manufactured, packaged, and sent to the finished goods area for release.
For example, goods may be released inadvertently before they are officially passed by QA or might be physically damaged in the warehouse.
They can pick up moisture or dirt or be dispatched to the wrong customer.
Sometimes, mistakes are made inside the warehouse. For example, defective goods returned from the marketplace may unintentionally be placed back into stock without quarantining and checking by QA.
Be aware of the following situations when preparing to dispatch a product:
– When dispatching, goods must be double-checked against the paperwork. Could you check the authorization on the requisition, the released status, the item name and code, and the container’s integrity?
– Only the correct quantity of products should ever leave the warehouse.
– Ensure that the oldest material is issued first (FIFO).
– Maintain appropriate dispatch records to ensure that each lot of products can be recalled if necessary.
iii. Preparation for transportation
After QA has officially released the finished batch, it may be moved from the Quarantine to the Released store (and labeled as such), making it ready for dispatch to other warehouses, hospitals, distributors, or retail outlets.
Incorrect transport can affect the quality of the product.
If the product is not protected from rough handling or adverse temperature conditions, it may be damaged and degrade or lose quality.
Other things could go wrong during transportation.
For example, the product may be counterfeited or substituted in the distribution chain, or it could be damaged by the environment in which it was transported.
It would be best to take special precautions since some products may be sensitive to vibration, changes in atmospheric pressure, or freezing or thawing.
iv. Picking and packing
When picking and packing the finished product, you should complete several checks of the product against the paperwork. For example:
– Verify the correct customer.
– Verify the product and its strength.
– Cross-check the dispatch order batch number.
– Verify the product is within expiry.
– Verify the dispatch order quantity.
– Ensure that packing instructions are correct.
v. Validation of transportation conditions
Once a product leaves the control of the warehouse, it can be impacted by mistakes during transportation and uncontrolled warehouse conditions.
Many medicines can degrade if heated or frozen, even for short periods.
These issues particularly apply to biological products, vaccines, and products labeled “Store 2°C —8°C. Do not freeze.”
For example, a product required to be stored at 2°C —8°C may degrade if it is stored at more than 1°C for any period of time.
If the product were frozen and then thawed, it would be tough to detect this physically, and it may make the product unusable.
Many companies validate their transport conditions to see if there are potential problems with long-distance transit.
Once conditions are validated, it is standard practice to include continuous temperature monitoring devices on all consignments that are moved long distances, mainly if they are sensitive to heat or cold.
These monitoring records are then used to check for no transport issues, keeping the product safe.
vi. Maintain all dispatch records
Issue invoices or packing slips for each delivery that should accompany the goods.
Records should be maintained that are clear and readily available. They should show the receipt and disposal of all products purchased and sold.
Keep all distribution records securely in a folder that should be accessible during audits by regulatory authorities.
Warehouse material handling on customer returns
There are many reasons customers can return sold products, such as you have oversupplied unintentionally or delivered incorrectly. Customers can return if the product appears to be faulty.
i. General rules for managing customer returns
You should develop a standard operating procedure for handling returned materials in your warehouse.
However, it is unsafe to assume that the returned stock could be suitable for re-sale.
There is generally no way to tell whether the return has been tampered with, degraded in transportation or during storage, or has been damaged in transit.
GMP rules require that the quality department examine all returns. If necessary, returned samples should be re-tested before they can be placed back into stock.
In some cases, if there is no way to tell if the returned products have degraded, you should destroy them instead of re-assigning them into stock.
Products earmarked to be reissued must be kept separate from the rest of the stock until the investigation is completed. They may be returned to stock only if the goods:
– Are in their original containers, unopened, and in good condition.
– Are known to have been handled and stored correctly.
– Have an acceptable shelf-life remaining.
– Have been assessed by the appropriate, authorized personnel.
You must keep records of all goods if they are returned to stock. In new deliveries, treat them as a first-in-first-out (FIFO) approach.
Medicines that are unfit for sale must be stored separately and disposed of securely (e.g., incineration).
ii. How to assess customer returns
The assessment of customer returns should be based on the goods’ nature and any special storage conditions they may require.
If necessary, take advice from the quality assurance team.
Follow the rules below while handling the return of non-defective stock:
– Place a limit on the return period (e.g., 3-5 days from customer receipt).
– No returns of “fridge lines.”
– Communicate the company’s policy to customers.
– Use a clear returns document that requires customers to include the product’s batch number and expiry date.
– Have detailed procedures for handling, locating, and identifying returns.
– Ensure warehouse staff are trained on the finished product return procedure and how to separate the returned goods from fresh stock.
– Ensure the material return procedure includes a detailed physical inspection and, if necessary, a laboratory test to verify that the goods are “safe” for re-stock.
– Maintain a product returns register showing dates, batch numbers, amounts, and conditions of the return. This register is needed for later investigation if there is a problem.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Warehouse material handling of product recalls
It’s almost inevitable that your company will experience a recall at some point.
GMP rules require that the warehouse (and the company) is prepared to manage an actual recall incident by having a validated recall procedure.
The procedure should detail how defective stock (recalled) should be returned, registered, counted, securely separated, and destroyed.
You must manage any product recall very carefully to protect patients and end-users.
Significant risks surrounding recalls include:
– The right people should be notified.
– The recalls should have been dealt with on time.
– Recalled product re-entering the supply chain.
Your warehouse should have a separate secured area for keeping recalled units.
You must have up-to-date systems and documentation that can successfully locate every part of the recalled product within the distribution chain or with customers.
In the event of a recall, quality assurance will agree with the regulators on the scope of the recall before the recall is triggered.
The recall decision will usually happen very quickly, so your warehouse must always be recall-ready.
Your company should assess the current quantity of stock in the marketplace and aim to recover 100% of available stock.
The notice will state that the recall must occur at a particular level in the marketplace.
Class (I) recalls occur at the consumer level. Class II or III recalls may arise at the retail or warehouse level.
For consumer-level recalls, there is usually a company hotline to assist customers.
In the event of a recall, defective products are collected at nominated warehouses.
It is progressively checked and counted, and the returns are recorded in a recall register.
As the recall progresses, the percentage of defective stock is monitored. If possible, the recall is continued until 100% of the stock is returned.
The detective product subjected to recall is eventually gathered in one central location in a separate, secure Recall store or location. The recalled material must always be in the main warehouse.
Once the recalled product has been secured, it should be securely destroyed so it cannot be re-entered into the marketplace.
You should develop secure product destruction procedures, and the physical destruction should be witnessed or otherwise verified.
Handling counterfeit products
Unfortunately, it has become prevalent for counterfeit medicines to make their way into the distribution chain.
Many of these products look almost identical to the original products, both in drug appearance and packaging.
These counterfeit products contain no active ingredients (APIs) and can harm patient health if consumed. They may even contain poisons.
Staff handling medicines in distribution or remote warehouse storage should be very alert for any product that looks suspicious or different from the usual product.
If anything seems odd, report immediately to warehouse Supervision and place the lot in quarantine for investigation.
Warehouse material handling equipment
In a typical warehouse environment, you have to use a number of tools and equipment.
The following is a list of equipment commonly used in a pharmaceutical warehouse for material handling.
Not all of these are necessary for your purpose. However, it is better to familiarize yourself with some of the equipment for work reference.
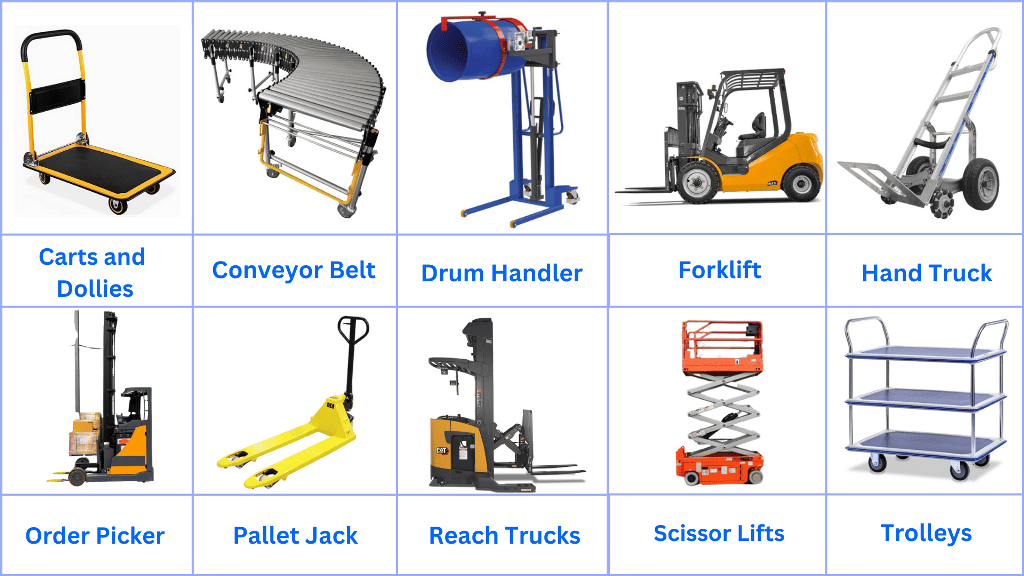
Forklifts: These are some of the most common pieces of equipment used for lifting and transporting heavy loads over short distances.
Pallet Jacks: These are used for lifting and moving pallets within the warehouse. They come in manual and electric versions.
Conveyor Belts: These are used for moving materials from one point to another within the warehouse, especially along the production line.
Shelving and Racking Systems: These are used for organizing and storing materials efficiently and making optimal use of space.
Carts and Dollies: These are used for transporting smaller loads or materials that need to be moved manually.
Order Pickers: These are specialized forklifts used for picking and transporting materials from racks at different heights.
Drum Handlers: These are used for lifting, transporting, and tilting drums containing liquid or powdered materials.
Platform Trucks: These are used for transporting bulky or heavy materials over flat surfaces.
Scissor Lifts: These are used for lifting personnel or materials to higher shelves or storage locations.
Stackers: These are similar to forklifts but are used for lifting and stacking pallets at greater heights.
Trolleys: These are manual carts with wheels used for transporting smaller items or materials.
Reach Trucks: These are a type of forklift designed to work in narrow aisles and are capable of lifting loads to greater heights.
Hand Trucks: These are L-shaped hand carts with wheels at the base and a small ledge to set objects on, flat against the floor when tilted back.
Dock Equipment: This includes dock levelers, dock boards, and dock plates that are used for loading and unloading materials from trucks.
Roller Conveyors: These are conveyors that use rollers to transport materials from one point to another.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Conclusion
Warehouse material handling in the pharmaceutical industry is a critical and highly regulated process.
Adhering to best practices and GMP rules can ensure quality, efficiency, and compliance in all aspects of warehouse management, from receiving incoming materials to delivering the right products to customers.
GMP guidelines expect us to take meticulous actions while receiving and issuing raw materials for production.
Errors can occur during these activities. If not caught in time, these errors can easily get away with the processing phase, resulting in defective products.
Warehouse errors, if not meticulously managed, can lead to undesirable outcomes for your company, such as batch rejections, customer returns, and recalls. Most critically, these errors can compromise patient safety, underscoring the importance of our collective responsibility in warehouse material handling.
Due to this, extreme caution must be exercised when handling materials, including when receiving raw materials, logging them in, storing them, sending them for testing, and dispensing approved raw materials.
Managing the movement of raw materials is just one aspect of warehouse material management.
The quality of the stored raw materials and finished goods can deteriorate over time due to mishandling and environmental factors such as uncontrolled temperature, humidity, and pest activities.
Prepare standardized procedures and train your staff to identify and correct mistakes in real-time.
Warehouse material handling also involves proper management of customer returns, counterfeit products, product recalls, etc.
Warehouse material handling demands accuracy, efficiency, and adherence to best practices.
By embracing continuous improvement through periodic reviews, updates to warehouse procedures, compliance with regulatory requirements, and investment in employee training, we can empower ourselves to ensure the highest standards in our industry.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.