Record Keeping and Record Management Practice in GMP
- Published on: Dec 16, 2020
Master processing instructions are probably the most critical documents in the plant. They provide the official registered, validated, company-approved instructions, and as such ensure product safety and compliance. Failure to follow the current instructions could lead to defective product, possible recalls, and harm to patients. Product manufactured not in compliance with the government-register details is against the law.
It is never permissible to hold unofficial photocopies of master instructions for convenience. One only approved copy of the current, approved master instruction should be released each time a batch is to be prepared, because from time to time these instructions are updated to a new version.
Importance of record keeping:
- The manufacturing and testing records (along with product retention samples) are all that remain once a batch is released.
- These records are the only real source of information on a batch after it has been released, so they must be accurate and complete.
- They provide legal evidence that the company followed GMP.
- There are many cases where in a legal dispute, the GMP-related records are used in court to verify that the company followed GMP.
- They are used to investigate product complaints/adverse events.
- Complaint investigation is a critical activity to protect patients’ health. If the records are not accurate and complete, incorrect conclusions may be made about the problem.
- They are used to investigate trends or problems.
- Often, production or product history need to be checked for trends. This can only be done if the records are accurate and complete.
- They are always reviewed during government audits.
- Auditors review records to ensure they are accurate and complete and follow GMP. They place importance on this review when forming opinions on company GMP standards.
Ideal practice of completing records:
Here are some simple rules for completing records:
- Records must be accurate.
- Records must be completed in real-time.
- Records must be legible.
- Records must be completed in a non water-soluble pen.
- Records must be stored to prevent loss or destruction.
- Never use whiteout or erasers.
- To correct data, cross out the incorrect data with one line, enter the new information, sign or initial it, and date it.
- Fill in all fields, even if you write “Not Applicable”.
GMP rules require that records are completed as the event occurs.
The reason for this is that if people rely upon memory at a later time, the record may not be accurate. The accuracy of batch records is critical to in-process controls, release of the batch to the market, and for later investigation if there is a customer complaint.
Leaving notes for other staff members is also a problem because they may get lost and are not part of the formal record system.
Recording information in the document itself:
- Only permanent ink, preferably blue or black, should be used to complete a document.
- Red ink might be used to highlight proposed changes in a document. All recorded information should be clear, legible, and accurate.
- Using a white correction fluid or writing over an entry to correct an error is not acceptable.
- If a correction is necessary, the incorrect information should be crossed out using one line.
- The correct information should be entered near the incorrect information accompanied by the initials of the person making the correction and the date the correction was made.
- If repeated information is required for several lines in a document, e.g., initials, no ditto marks (“) or arrows (¯) should be used. Initials should be entered each time they are required.
Recording data
All data should be recorded directly onto the production batch record, appropriate record, logbook, form or worksheet. Errors, which are corrected based on information contained in another source document, must be referenced or the source document must be attached. Personnel who verify calculations must do so independently.
Numerical data
Numerical results must be reported using the same number of significant figures as required by the specification or standard.
Required significant digits to four places: 23.42
Wrong: 23.424
Wrong: 23.4
Analytical data rounding should follow the appropriate site SOP. Rules for rounding other numerical data are found below:
Rules for rounding numbers
For numerical data other than analytical data:
- If the digit to the right of the digit to be retained is less than 5, do not round up to the next higher digit.
Example: 3 significant digits required
Result: 23.74 Round to: 23.7
- If the digit to the right of the digit to be retained is 5 or greater, round up the result to the next higher digit.
Example: 3 significant digits
Result: 23.77 Rounded: 23.8
Comments made in the Batch Records
Comments made to clarify data or statements must be initialed and dated. Extended downtime during a production operation that prevents in-process checks from being performed must be noted with an explanation on the batch record, i.e., “in-process checks delayed due to shift change” or “machine down for repairs preventing in-process checks from being performed”.
Errors, which are corrected based on information contained in another source document, must be referenced or the source document must be attached.
Reasons for problems must be explained in detail, initialed, dated and written on the corresponding pages.
Examples:
- Writing “line down” (indicating a production line is down) on a batch sheet is not sufficient. Briefly explain the reason the line is down.
- Writing “problem” on a batch sheet is not sufficient. Briefly provide information that clarifies what the problem is.
If a source external to the batch record is used to change data or statements, explain the source of information. Only authorized personnel are allowed to review and approve batch records.
Corrections to batch records should be made by the person who made the original entry. If this is not possible (e.g., the person no longer works at the site), efforts should be made to reconstruct the data using other viable or appropriate data sources.
Different sites may have different documentation rules regarding batch records.
Non-applicable pages of information
If a complete sheet of information on a document is not required by the process, a single diagonal line may be drawn from corner to corner. On the line “N/A” (Not Applicable) should be entered with the person’s initials and date. If there are multiple pages that are not used, the site should have a defined procedure outlining the actions to be taken.
If critical data are not recorded, a deviation report must be initiated. The deviation report form number must be included on the applicable page(s) of the batch record. Critical data include equipment readings, time, temperature, pressure, and in-process checks. If changes or corrections are necessary to the batch record, they must be explained and approved by quality. Formal documentation, i.e., a memo or signed change request form, must be kept for each change.
Types of documentation systems that are audited
Documentation systems that should be routinely audited are:
- Equipment assembly, cleaning and use log.
- Component, drug product container, closure, and labeling records.
- Master production and control records.
- Batch production and control records.
- Production record review.
- Laboratory record systems including electronic data systems and analytical methods.
- Distribution records.
- Annual Product Reviews
- Complaint files.
- Validation and qualification systems of processes and equipment.
- Equipment calibration and maintenance records.
- Environmental monitoring records.
- Training records.
Components of documentation systems
All documentation systems should have approved and established SOPs that govern how the particular documentation system is managed. The topics that should be included, at a minimum are:
- Document control and distribution.
- Approving bodies and date of approval.
- Timeline for review and revision.
- A tracking and follow-up method.
- Assurance of training regarding documentation.
- A description of the purpose of the system.
- Storage and retrieval information.
- Directions for handling atypical occurrences within the system.
Documentation may consist of logbooks, worksheets, electronic data, highly controlled paper documents, equipment readout or charts.
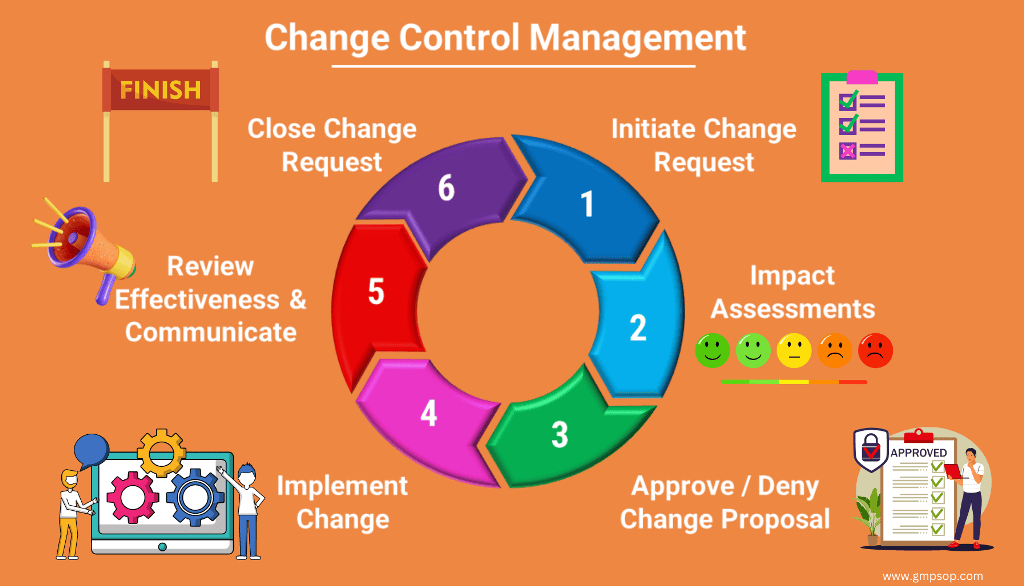
Revision of documents
GMP document revisions should be properly controlled through a formal revision system as part of the document management system. Documents should be approved, signed and dated by appropriate and authorized persons, as defined in procedures approved by Quality Assurance. As part of the document management system there should be a method in place to assure that GMP documents are revised regularly to comply with the GMP requirement that states that all documents should be complete and accurate. When revised documents are issued, there should be a method in place to promptly remove superseded revisions from the area. Only official records and procedures should be used by personnel.
If approved documents (e.g., validation reports, released batch records, etc.) need to be amended, only appropriate personnel who have proper authorization may amend the documents. There should be a system in place to manage proposed amendments to approved documents.
The site should have a record retention schedule for different types of documents. All records associated with a manufacturing lot should be retained for a minimum of one year after the drug product expiration date. Validation records or other records that are not specific to batches or lots may have to be retained longer.
Records may be retained as original copies or true copies, e.g. microfilm, scanned copies, etc. Documents may be stored off site but must be easily retrievable. Original raw data of manufacturing and the laboratory should be retained according to site procedures.
Electronic data
If data is recorded in electronic systems, detailed procedures relating to the system in use should be available and the accuracy of the records should be checked. Only authorized persons should be able to enter or modify data in the computer and there should be a record of changes and deletions; access should be restricted by passwords or other means and the result of entry of critical data should be independently checked. Batch records and other critical documents electronically stored should be protected by back-up transfer on magnetic tape, microfilm, paper or other means. It is particularly important that the data are readily available throughout the period of retention.
Key points about documentation and record keeping:
- A good documentation system is the cornerstone of a GMP compliance system.
- All personnel must follow the written and approved documents in the day-to-day operation of the manufacturing facility.
- It is the responsibility of management to provide a documentation system that conforms to the GMP requirements, and then train the users in the requirements of the system.
- It is your responsibility to understand the documents and use them correctly.
- When you sign or initial a record saying that you carried out a process, you are certifying that the process was carried out according to the written and approved procedures.
- When you sign or initial a checking step in a record, you are saying that you observed what was done, and can verify that it was done correctly.
- Remember: if it’s not documented, it wasn’t done.
It is only by having a documentation system that everyone complies with, that we can demonstrate that we have manufactured a quality product.
Summary
- A good documentation system is the cornerstone of a GMP compliance system.
- All personnel must follow the written and approved documents in the day-to-day operation of the manufacturing facility.
- It is the responsibility of management to provide a documentation system that conforms to the GMP requirements, and then train the users in the requirements of the system.
- It is your responsibility to understand the documents and use them correctly.
- When you sign or initial a record saying that you carried out a process, you are certifying that the process was carried out according to the written and approved procedures.
- When you sign or initial a checking step in a record, you are saying that you observed what was done, and can verify that it was done correctly.
It is only by having a documentation system that everyone complies with, that we can demonstrate that we have manufactured a quality product.
Remember: if it’s not documented, it wasn’t done.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
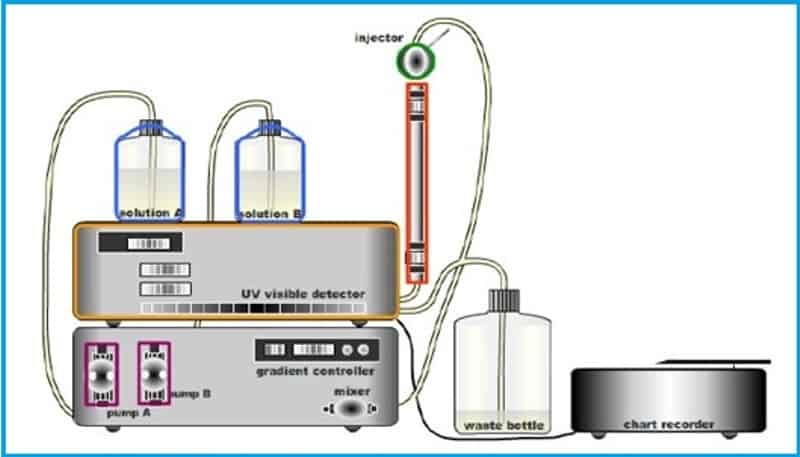
Chromatographic systems used in Pharmaceutical Laboratories
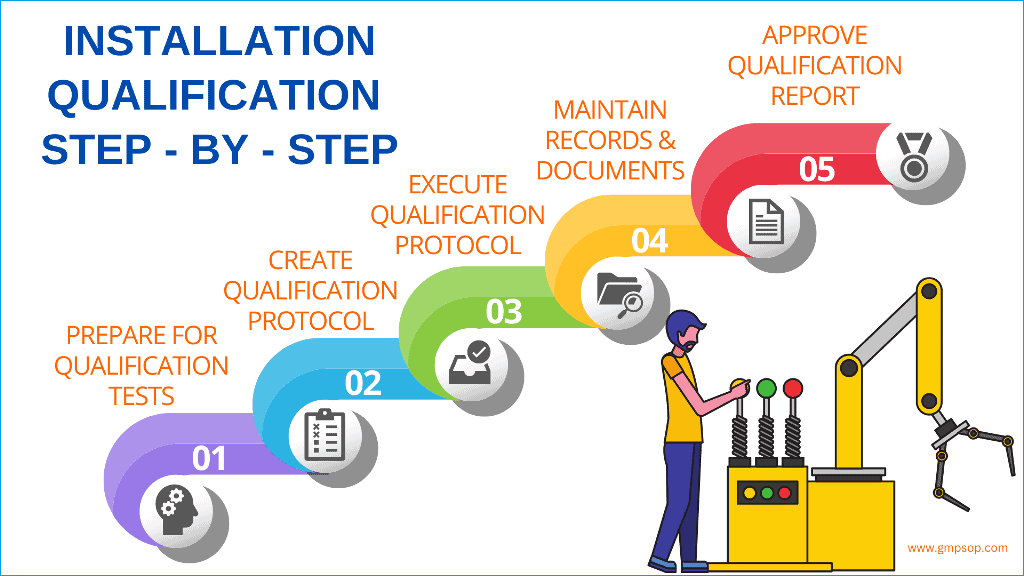
A step-by-step guide to successful installation qualification (IQ)
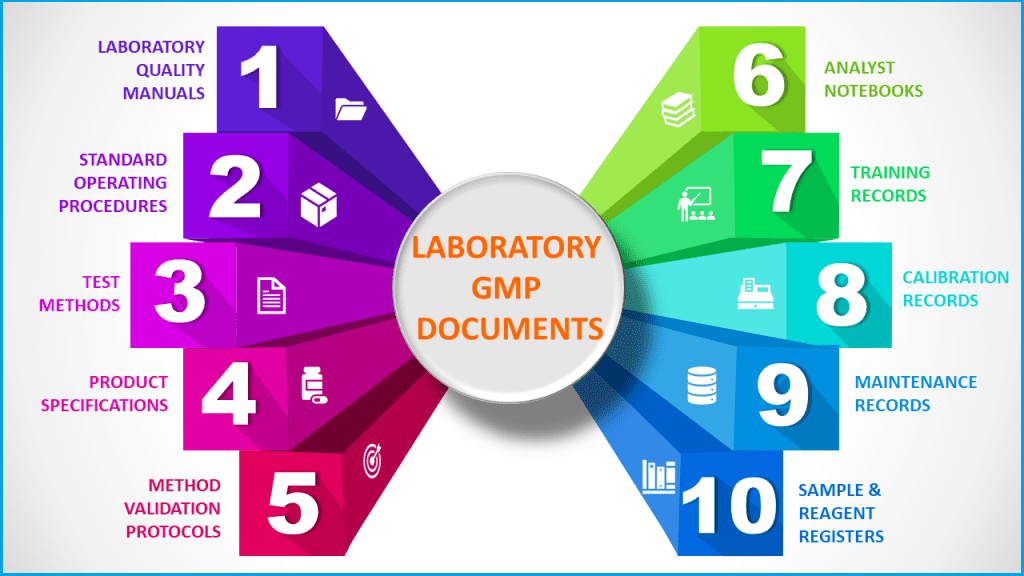
Typical GMP documentation in a quality control laboratory