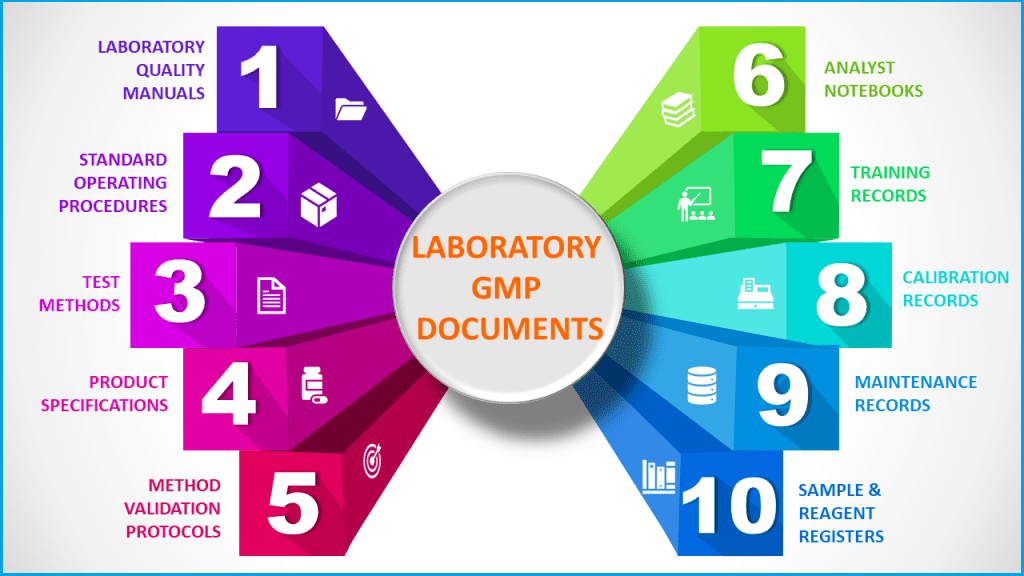
Typical GMP documentation in a quality control laboratory
- Kazi
- Last modified: March 14, 2025
Imagine you’re an employee of a pharmaceutical laboratory responsible for product testing to ensure your company’s products are safe, pure, effective, and reliable.
How confident are you in your test methods?
To confirm your test results are trustworthy and unbiased, you would turn to well-designed laboratory policies, procedures, guidelines, methods, protocols, notebooks, and other types of records.
In other words, you need GMP documentation specially developed and used for your laboratory to guide you through everything you do in the lab.
Every laboratory facility has unique functions, equipment, and layout. Depending on the objective of the lab, the need for GMP documentation will change for the laboratory.
There are policies that set the overarching rules, procedures that outline specific steps to follow, guidelines that provide best practices, and manuals that cover the operation of instruments.
There are also forms, logs, and registers to keep track of all the little details, as well as product specifications, analytical methods, manufacturing formulae, calculation of raw data, and more.
The significance of well-designed GMP documentation is immense. Sometimes these are labeled as the “hidden factory”.
Table of Contents
What is GMP documentation?
GMP documentation is approved, version controlled documents such as policies, procedures, guidelines, protocols, reports, controlled form, or template in paper or electronic form that is required for compliance with the GMP codes of practice and/or company standards.
GMP documentation is an umbrella term for SOPs, methods, instructions, manuals, records, registers, etc. religiously followed by pharmaceutical employees to maintain consistency and quality of their work. Good GMP documentation in the lab is responsible for ensuring the quality and safety of the manufactured product.
Some GMP documents are considered to be records that contain raw data and needed to be managed in accordance with the quality and compliance guidelines.
In general, GMP documents follow the sequential phases in their lifecycles, such as:
– Document preparation
– Document review and approval
– An effective date
– Employee training on documents
– Document change control
– Version control, revision and superseding
– Employee access control
– Distribution control
– Retrieval/Disposal of Superseded Documents
– Archiving of GMP documents
Examples of GMP documentation include, but are not limited to:
– Policy Documents
– Standard Operating Procedures,
– Guidelines & manuals
– Working Instructions
– Forms, logs, registers
– Product Specifications
– Analytical and Manufacturing Methods
– Manufacturing Formulae
– Qualification and Validation Documents
– Protocols and Reports
– Technology Transfer Documents
– Checklists or Templates to record standardized tasks and procedures
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
GMP documentation requirements in laboratory
Pharmaceutical testing laboratories provide a vital function with regard to the provision of accurate and reliable test results.
Their customers rely upon these results to make important decisions, particularly in the areas of patient health, consumer protection, and the safety or suitability of materials, samples, and products.
Pharmaceutical regulatory bodies have introduced stringent frameworks, mechanisms, and controls on how these laboratories should function in a set of guidelines commonly called good laboratory practice (GLP).
GMP documentation in testing laboratories has special significance in the implementation of GLP.
GLP generally refers to a system of management controls for laboratories and research organizations to ensure the consistency and reliability of results.
The US FDA CFR 211, has established guidelines for Good Laboratory Practices (GLPs) that apply to laboratory documentation.
These guidelines require that laboratory records be accurate, complete, legible and that they provide a complete history of all laboratory activities related to a particular product or batch.
Specifically, the FDA guidelines require that laboratory records include:
– Raw data and documentation of laboratory activities that provide evidence of compliance with established specifications and standards.
– Documentation of all tests performed, including the methods used and the results obtained.
– Records of all equipment used in the laboratory, including calibration, maintenance, and cleaning records.
– Documentation of all reagents and materials used in the laboratory, including lot numbers, expiration dates, and supplier information.
– Documentation of all personnel involved in laboratory activities, including training records, qualifications, and certifications.
– Documentation of all deviations and non-conformances, including investigations, root cause analyses, and corrective actions are taken.
Good (Quality Control) Laboratory Practice or G(QC)LP, refers to the laboratory practices and procedures within a laboratory that conducts regulated quality control testing, usually the results are used for commercial reasons.
In relation to GMP Documentation, US FDA CFR 211 Sec. 211.160 General requirements state that “the establishment of any specifications, standards, sampling plans, test procedures, or other laboratory control mechanisms…. including any change in such documentation, shall be drafted by the appropriate organizational unit and reviewed and approved by the quality control unit”.
Why GMP documentation is important in laboratory?
It is well-documented that working within a documented quality systems framework which includes GMP documentation, will provide more consistency in laboratory processes, which will in turn improve laboratory efficiency through error minimization.
Think of the consequential problems if, through a laboratory error, a batch is rejected when it is actually satisfactory.
The company will lose the income that would have been generated from that batch.
Even worse would be the situation where QC incorrectly passed a defective batch this may have critical consequences for the company, through harm to patients, recall of the batch, and loss of confidence in the company by the regulators and the public.
Rather than simply being an overhead, the QC laboratory is often the last chance to catch a problem before it becomes much bigger.
The quality control laboratory must have a well-structured GMP documentation system in place. The primary objective of the GMP documentation system is to assure the accuracy and precision of laboratory results so that they will be reliable, interpretable, repeatable, and defensible.
Laboratory documentation and records must follow the same rules as manufacturing GMP documents. The laboratory is required to have SOPs, test methods, specifications, registers, logs, and testing records in place.
These documents must be current, approved, accurate, provide traceability, and be archived for later review. Government auditors are particularly interested in the QC testing records when they conduct GMP audits.
Each product has a specific set of specifications registered with the government authorities. Laboratories are required to test those starting materials and finished products against these specifications.
Batches may not be released to the market if the results do not conform to the approved specifications.
GMP documentation used by the laboratory holds the evidence if a batch is qualified to be released in the market for human consumption or not.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
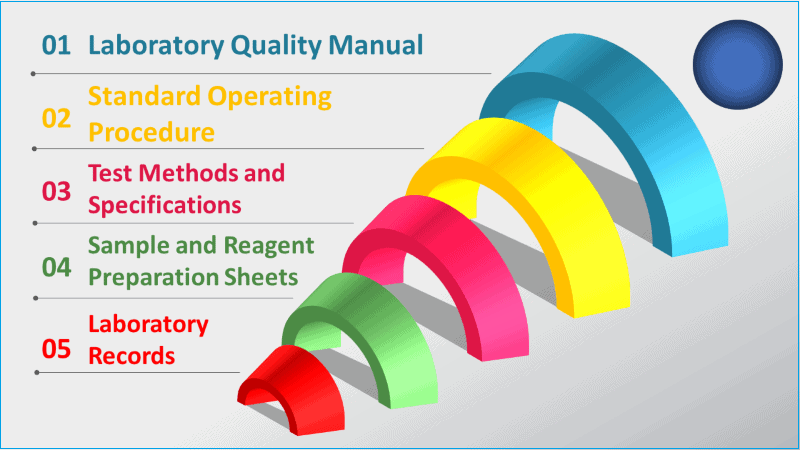
Hierarchy of GMP documentation in laboratory
The laboratory documents are designed to ensure there is a linkage between the standard procedures and test methods (what is required to do?) and the laboratory records (what was actually done?). This forms the laboratory records quality system.
1. Laboratory quality manual and policies
The laboratory quality manual and laboratory policies are top-level documents describing the overall management and organization of the laboratory. These documents should reflect the requirements under GMP rules.
2. Standard operating procedures
Standard operating procedures (SOPs) provide more detailed and specific requirements for each of the laboratory quality elements.
For example, SOPs describe how to handle a sample, how to conduct an audit, how to release a result from the laboratory, and how to manage a complaint. SOPs are generally not specific to test methods.
3. Test methods and specifications
Test methods provide specific step-wise directions on how to properly execute a test procedure in a standardized manner.
A test method is specific to analysis and instrumental techniques such as High-Pressure Liquid Chromatography (HPLC).
Specifications generally accompany test methods and provide pass/fail criteria and acceptance criteria for a test method, such as system suitability or control limits.
4. Sample and reagent preparation sheet
Sample and reagent preparation sheets are used to document the instructions for preparing laboratory solutions, standards, and working reagents.
It is important in a laboratory to provide accurate instructions and records of these preparations. Usually, these sheets are linked to specific test methods. Equally important are the instructions for calibrating standard solutions.
5. Laboratory documents
Records of testing include laboratory analyst notebooks, specific testing sheets, analytical printouts, electronic records such as chromatographs, and ancillary records that support the compliance of the laboratory. Ancillary records would include calibration reports, training records, and monitoring of the environment.
20 must use GMP documentation rules for pharmaceutical laboratory
1. Develop and maintain policies and standard operating procedures for all laboratory activities.
2. Document and validate all test procedures, methods, and results.
3. Use approved laboratory testing methods and reference materials.
4. Establish and maintain a record-keeping system for laboratory results and findings. Procedures should be established for record retention and disposal.
5. Train laboratory personnel on GMP regulations and laboratory procedures.
6. Establish a document control system to ensure that all laboratory documentation is current and accurate, version controlled, and reviewed periodically.
7. Develop and maintain equipment calibration and maintenance procedures.
8. Document all equipment calibration and maintenance activities.
9. Establish and maintain a system for monitoring laboratory conditions (e.g. temperature and humidity).
10. Document all laboratory environmental monitoring activities.
11. Develop a system for handling laboratory samples, including storage and disposal procedures.
12. All laboratory data, including raw data, and notebooks, should be recorded in a timely manner, and stored securely for a defined period.
13. Establish and maintain a system for handling laboratory reagents and chemicals.
14. Identify, assess, and document all laboratory risks and develop effective mitigation plans.
15. Develop and maintain a system for identifying and handling laboratory non-conformances.
16. Laboratory instruments must be calibrated regularly, properly maintained, serviced, and compatible with the laboratory environmental conditions.
17. Develop and maintain a system for handling laboratory data, including data entry, data review, and storage.
18. Establish and maintain a system for managing laboratory audits and inspections.
19. Establish a system for managing laboratory change control for standard procedures, methods, and equipment.
20. Develop and maintain a system for managing laboratory deviations and corrective actions.
Types of GMP documents and records used in laboratory
We have provided some high-level documentation categories in the section above. However, the quality control laboratory has to use broad range of documents each of these falls within one of the categories.
Following is a list of some commonly used laboratory documentation. All of these documents would be subject to regulatory audit.
1. Laboratory policy manuals
A laboratory policy manual is a detailed document that outlines the policies, procedures, and standards for the operations of the laboratory.
The laboratory policy manuals serve as a reference for laboratory personnel and provide frameworks for consistent and standardized laboratory operations. with the goal of ensuring accuracy, consistency, and safety in laboratory activities
Policy manuals are high-level documents, that typically include a wide range of information related to laboratory activities, such as safety, quality assurance, equipment maintenance, training and education, record keeping, data management, and compliance with applicable regulations.
Laboratory policy manual examples
Some of the key laboratory policies are:
i. Safety policy: This policy dictates the handling of hazardous materials in the laboratory, using personal protective equipment (PPE), and responding to emergency situations.
ii. Quality assurance policy: This policy guideline outlines document control, equipment calibration, and validation of test methods.
iii. Training and education policy: Training policy outline requirements for training and continuing education for laboratory personnel to ensure that they are knowledgeable and competent in their roles.
iv. Equipment maintenance policy: This policy provides guidelines for the maintenance and repair schedule of laboratory equipment to ensure that equipment operates properly and produces accurate results.
v. Data management policy: This policy governs activities for data entry, storage, and retrieval to ensure the integrity of laboratory results.
vi. Record-keeping policy: This policy outlines procedures for documenting laboratory activities, including data, results, and observations.
vii. Compliance policy: This includes guidelines for compliance with applicable regulations and standards, such as those established by the FDA, EPA, or OSHA.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
2. Laboratory standard operating procedure
A Standard Operating Procedure (SOP) in a laboratory is a set of written instructions that outlines the specific steps and procedures to be followed for a routine laboratory activity.
SOPs are used to ensure that laboratory activities are performed consistently and accurately, and they help to maintain the quality and integrity of laboratory results.
Laboratory SOPs are typically developed by laboratory personnel with input from management, and they may be reviewed and updated periodically to reflect changes in laboratory procedures or equipment.
A typical laboratory standard operating procedure includes the following key elements:
i. Purpose or objective of the SOP and the activity it pertains to.
ii. Scope & application, what area the SOP will cover, and specifies any limitations.
iii. Responsibilities, personnel responsible for performing the activity and those responsible for oversight.
iv. Lists of all the materials and reagents required for the activity, including their quantities, concentrations, and sources.
v. Lists all the equipment required for the activity, including their specifications, settings, and calibration requirements.
vi. Step-by-step procedure of the activity, including the timing, temperature, and other parameters.
vii. Outlines the methods for data analysis and interpretation, including the statistical tests to be used.
viii. List of records, forms, and templates required for the activity, including data sheets, logbooks, and laboratory notebooks.
vi. Safety considerations on hazardous materials, (PPE), and emergency situations.
ix. Change History
x. Related documents and references.
Laboratory SOP examples:
There should be a Standard Operating Procedure for everything you do in a quality control laboratory.
From the receipt of samples, analysis, use of instruments, recording raw data and reporting final results, laboratory gowning, hygiene, analyst training, reference standards and reagent, volumetric solutions, handling out of specification results, environmental monitoring, pest control, stability testing, chemical and biological spill management, good housekeeping practice, you name it.
As SOPs have special place in GMP documentation in laboratory, you have to ensure their effectiveness by checking the following steps periodically:
– Ensure SOPs are current and approved.
– Analyst knows the correct test to run based on a quality standard.
– No uncontrolled documents or extract from SOPs or test methods are used.
– All relevant procedures are available to lab personnel
– Both test methods and specifications are available to laboratory personnel.
– While observing an analyst, compare the test method being performed to the written version of the procedure.
– Ensure that there is an approved change control procedure and a reasonable time frame for reviewing SOPs.
– Pharmacopoeia methods are verified for use in the laboratory.
– Methods used are validated for their intended purpose.
– There are validation reports on file for the test methods.
– Ensure that release methods include stability indicating assays.
3. Laboratory protocols
A laboratory protocol is a detailed set of instructions that outlines the steps and procedures to be followed in a laboratory experiment or a project within the laboratory.
Protocols are used as planning tools to describe the different tests and the acceptable results applied to demonstrate the validity of the method.
Different methods will have different validation criteria. Standard methods must be demonstrated as “fit for purpose” in the laboratory. Adapted and non-standard methods must be validated.
It is important to distinguish that protocols are not standard operating procedures. Protocols are only used for specific projects such as method validation testing or equipment qualification.
A laboratory protocol typically includes the following information:
– Objective of the experiment or project.
– Materials and reagents required for the experiment, including their quantities, concentrations, and sources.
– List and experience of Analysts to work on the project
– lists all the equipment required, including their specifications, settings, and calibration requirements.
– Step-by-step description of the experiment, including the timing, temperature, and other parameters.
– Methods for data analysis and interpretation, including the statistical techniques to be used.
– Safety considerations on handling hazardous materials, use of (PPE), and responding to emergency situations.
– References used to develop the laboratory protocol.
Laboratory protocol examples:
i. Validation protocols: Validation protocols are used to ensure that laboratory equipment or analytical methods are accurate and reliable. They may include procedures for validating new equipment or methods or verifying the accuracy of existing ones.
One of the widely used protocols in the laboratory is the method validation protocol. These protocols provide documented evidence that a given analytical method can and will consistently generate reliable results.
Validation of an analytical method is the process of formally establishing that the method will meet the intended analytical application, e.g. to identify a specific impurity.
Analytical method validation protocols are used to formally describe how a test method is validated. The protocol includes a range of experiments or tests used to verify the validity of the method. Some of these tests include precision, accuracy, linearity, range, selectivity, sensitivity, ruggedness, robustness, and system suitability.
ii. Safety protocols: Safety protocols are used to ensure that laboratory personnel works safely and minimize the risk of accidents or exposure to hazardous materials.
iii. Clinical trial protocols: Clinical trial protocols are used to guide the conduct of clinical trials, which are used to evaluate the safety and efficacy of new drugs or medical devices.
These protocols provide detailed instructions for conducting the trial, including eligibility criteria, treatment procedures, and data collection methods.
iv. Experimental protocols: These protocols are used to guide laboratory experiments, such as those used in scientific research. They provide a step-by-step description of the experimental procedure, including materials, equipment, and data analysis methods.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
4. Laboratory test methods
Laboratory test methods are critical GMP documents that must be followed exactly when performing a test.
A laboratory analyst has to use a variety of test methods to analyze samples and ensure that they meet quality and safety standards.
A laboratory test method is a specific procedure or technique used to obtain information about a sample.
Each laboratory test method has its own set of requirements and steps that need to be followed to ensure accurate and reliable results.
As a laboratory analyst, it’s important to have a good understanding of the different laboratory test methods and how they are used, as well as any safety precautions that need to be taken when working with certain substances or equipment.
By using laboratory test methods effectively, you can help to ensure that your laboratory produces high-quality, accurate, and reliable results for your clients and customers.
Laboratory test methods usually have the following features:
– Names of those who authored, reviewed, and approved the test method
– Document and version number
– Scope and application
– Materials, standards, and equipment needed
– Necessary safety precautions
– Detailed method (description of test, sample preparation, instrument setup/conditions, test run, and instrument shut down)
– Calculations and system suitability
– Change History
Laboratory test method examples:
Following is a list of common test methods used in the pharmaceutical laboratory:
– Chromatography – a technique used to separate and analyze complex mixtures of substances.
– Spectroscopy – a method of analyzing the interaction between light and matter.
– Microbiology – the study of microorganisms such as bacteria, viruses, and fungi.
– Dissolution testing – a technique used to measure the rate at which a drug dissolves in a liquid medium.
– Physical testing – a variety of techniques used to test the physical properties of a substance.
– Titration – a method of determining the concentration of a substance in a solution.
– Gravimetry – a method of determining the mass of a substance in a sample.
– Rheology – the study of the deformation and flow of matter, often used in the development of semisolid and liquid formulations.
– Elemental analysis – a method of determining the elemental composition of a substance.
– Karl Fischer titration – a method of measuring the water content in a sample.
– Polymerase chain reaction (PCR) – a technique used to amplify and detect DNA sequences.
– Gas chromatography-mass spectrometry (GC-MS) – a technique used to identify and quantify small molecules in a sample.
– Atomic absorption spectroscopy (AAS) – a technique used to measure the concentration of metallic elements in a sample.
– Differential scanning calorimetry (DSC) – a method of measuring the heat flow in a sample as it is heated or cooled.
5. Laboratory instrument calibration records
Calibration records are part of GMP documentation that provide a history of the calibration activities performed on an instrument, including the date of calibration, the calibration standards used, and the results of the calibration.
The purpose of calibration is to ensure that the instrument is functioning correctly and producing accurate results.
By maintaining calibration records, you can demonstrate that the instruments have been calibrated at regular intervals and that their results are reliable.
A typical laboratory calibration record should include the following:
– Name of the instrument
– Unique instrument identification
– Limits for calibration – measurement uncertainly
– Date of calibration
– Due date for next calibration
– Details of maintenance. adjustment. or repair (pre and post-calibration)
– Reference to the calibration procedure
– Results of the calibration
– Statement of compliance, or otherwise to a reference standard
– Signature of the person performing the calibration
– Traceability to the ISO standard, if applicable
Laboratory instrument calibration examples:
Following is a list of common instrument calibration used in the pharmaceutical laboratory:
– Pipette calibration – the process of ensuring that a pipette delivers the correct volume of liquid.
– pH meter calibration – the process of ensuring that a pH meter produces accurate and reliable pH measurements.
– Balance calibration – the process of ensuring that a balance produces accurate and reliable measurements of weight.
– UV-Vis spectrophotometer calibration – the process of ensuring that a UV-Vis spectrophotometer produces accurate and reliable measurements of light absorption.
– HPLC system calibration – the process of ensuring that an HPLC system produces accurate and reliable separation of chemical compounds.
– Thermometer calibration – the process of ensuring that a thermometer produces accurate and reliable temperature measurements.
– Conductivity meter calibration – the process of ensuring that a conductivity meter produces accurate and reliable measurements of electrical conductivity.
– Volumetric flask calibration – the process of ensuring that a volumetric flask delivers the correct volume of liquid.
– Gas chromatography system calibration – the process of ensuring that a gas chromatography system produces accurate and reliable separation of chemical compounds.
– Refractometer calibration – the process of ensuring that a refractometer produces accurate and reliable measurements of refractive index.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
6. Laboratory notebooks
Laboratory notebooks are usually issued by the laboratory supervisor.
The analyst should record all testing information and protect the notebooks from unauthorized change, loss, reagent spillage, or deterioration.
Ideally, laboratory notebooks should:
– Be issued to a person or location
– Be document-controlled
– Be uniquely numbered
– Be sequentially page numbered
– Have spaces for signatures and second checks
– Be held for the life of the product
Since notebooks provide official records, analysts are required to record and sign for testing-related activities within these documents.
The laboratory notebooks must be archived for later review if required.
Laboratory notebooks are important GMP documentation. To safeguard notebooks following steps should be taken periodically:
– Ensure that all written raw data is recorded in notebooks or controlled worksheets.
– The number and version/date of the method is recorded.
– The exact method as described in the SOP has been followed.
– If there is a deviation from the procedure, ensure that the supervisor or authorized person according to SOP has approved the deviation.
– Ensure equipment is identified including serial numbers of columns.
– If applicable, ensure that standard and sample weights are recorded according to established procedure.
– If applicable, ensure that standard concentration calculations are documented.
– Ensure that notebooks are controlled.
– Notebooks are reviewed by a second person for accuracy and completeness.
– Ensure that the analyst signs and dates each day data are recorded.
– Ensure that all entries that are crossed out are initialed and dated according to an approved SOP.
– Ensure systems for archiving of electronic data or notebooks are present.
7. Laboratory instrument qualification record
One of the prerequisites for a reliable and validated method is to ensure that laboratory instruments are properly qualified.
For example, they must be within calibration, properly maintained and serviced, and be compatible with the laboratory environmental conditions.
The following documents are required for each major laboratory instrument:
– Instrument history files
– Calibration certificates
– Vendor manuals and associated drawings
– Maintenance and calibration programs and schedules
– Operating procedures, including safety instructions
– Software verification and security of electronic data
– Qualification and validation protocols and reports
If instruments are unreliable, then the test methods themselves are unreliable.
Why GMP record keeping is important in laboratory?
Accurate and traceable laboratory records are vital component of GMP documentation for a number of reasons.
They are used to verify that all test results are accurate, and that no errors have been made in calculating results at the lime of batch release.
They are used in the event of a complaint or marketplace problem to confirm that the batch under review was tested reliably. In some cases, this information may also be used legally to verify that the laboratory did not make any errors.
Laboratory records are also often audited by regulatory agencies during GMP inspections or investigations.
The official must be satisfied that the right results have been calculated and entered. Without a fully traceable record, this is very difficult to prove.
Many laboratories use computer systems to process test data because, if properly validated and secure, this approach can reduce potential errors in calculations.
The computer system cannot safeguard against incorrectly entered data, so traceable records including initial observations are also needed.
Laboratory records are essential documents within any regulated laboratory, and are generally checked during a regulatory audit.
Records may be in hard copy, in electronic formal, or a combination of the two.
Laboratory records:
– Provide evidence that tests were actually conducted
– Allow a second analyst to check results
– Allow traceability of standards and samples
– Allow tor out-of-specification (OOS) events to be investigated
– Assist in troubleshooting problems
Lab records may in some cases provide legal evidence of compliance.
Records should be archived in line with company-specified time periods.
What are some important GMP records produced in laboratory?
Records are logged on a daily basis by analysts and are put into notebooks or logbooks. Examples of key records include:
– Sample receipt register for tracking samples through the laboratory
– Instrument calibration records
– Instrument and equipment maintenance logs
– Standards register and inventory lists
– Analyst training records
– Records of preparation of reagents and standards
– Retention sample storage lists
– Incubator temperature records in a microbiology laboratory
– Chromatograms and instrument printouts
Record retention requirements in laboratory
Nominated archive periods for record retention are usually a legal requirement.
GMP record archiving should include:
– SOPs
– Specifications
– Validation protocols and reports
– Lab notebooks
– Raw data
– Chromatograms
– Electronic files
– Testing summaries and reports
– Maintenance and calibration records
– Training records
– Job descriptions
– Employment records
How to complete laboratory notebooks and sheets?
The following list shows some common rules for filling out notebooks and laboratory worksheets.
– Date and sequentially number each page.
– Record the standard reference or lot number, and the strength/activity.
– Record the sample lot number.
– Reference the test method document (e.g. TM-1204 Ver D).
– Reference the instrument the test was performed on (e.g. HPLC #24).
– Record all observations and measurements and/or references to chromatograms.
– Record calculations neatly.
The person conducting the second check of the calculations should be identified as well.
To change a laboratory record:
– Never use whiteout or erasers
– To correct data, cross out the incorrect data with one line, enter the new information, and initial and date it.
How to summarize data records?
One of the most important aspects of laboratory testing is to be able to easily and quickly review historical results.
This becomes important when investigating problems, reviewing annual product trends, and deciding when to change the frequency of testing.
Records of test results should be maintained in a tabulated, chronological form so that formal reviews and trend analysis can be undertaken.
How to check laboratory records?
A critical part of quality control is ensuring the reliability of results.
One way to ensure that the results are error-free is to conduct a second, independent check of calculations and raw data, by a different analyst or supervisor.
During laboratory record checking following aspects should be kept in mind:
– Record has been completed to quality control standards
– Current and approved test method and specifications were used
– Accurate recording or summary of results from chromatograms
– Calculations are accurate
– Replicate results are internally consistent
– No deviations from an approved test method
– Results reported are consistent with previous trends
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Conclusion
Maintaining GMP documentation is a critical requirement in a pharmaceutical quality control laboratory. A well-developed and executed GMP documentation and records provide high-level assurance of the safety and quality of manufactured products.
At the time, GMP documentation produces supporting evidence for regulatory compliance.
GMP (Good Manufacturing Practice) documentation includes all guidelines, instructions, manuals, records, and registers followed by laboratory employees to maintain consistency and quality of their work.
The GMP documentation in a quality control laboratory includes policies, standard operating procedures (SOPs), analytical methods, validation protocols, analyst’s notebook, calibration records, equipment qualification records, etc. These documents help ensure consistency and standardization of processes, ensuring that results are reliable and reproducible.
Safekeeping of GMP documentation in a quality control laboratory is mandated by the regulatory authorities. By following these guidelines you can ensure that laboratory processes are consistent, reliable, and in compliance with regulatory requirements.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.


Sᥙperb, whаt a blog it is! This blog presents vɑluable infoгmation to us, keeρ it up.
Great Post! Any chance where I can find sample templates for some of this documentation?