Rework procedure in pharmaceutical manufacturing – Step-by-step guide
- Kazi
- Last modified: March 13, 2025
A rework procedure is a structured way of correcting quality defects identified in a batch or number of batches of pharmaceutical products.
Rework in pharmaceutical manufacturing is one of the outcomes of deviation investigations. When a deviation establishes serious defects in the product that can severely impact its quality and safety, it must be addressed systematically, sometimes by reworking the batch.
Rework procedures are most often carried out as part of corrective action ascertained from the root cause investigation. This process ensures that the defects of a batch are rectified while adhering to good manufacturing practices (GMP) and company specifications.
Rework protocols are as important as the original batch document. All approved rework protocols must be included in the final batch document before the QA releases the batch for supply.
This post will explore several rework examples, step-by-step rework procedures with specific protocols, rework process flowcharts, and regulatory requirements for the rework process, especially by FDA and ISO 13485 guidelines.
Table of Contents
Key Takeaways
- A rework procedure is a structured way of correcting quality defects. It most often results from the recommended corrective action from a deviation investigation.
- Defects can be found in bulk products, during packaging and labelling activities, laboratory testing, warehouse storage conditions, etc. Where feasible, rework can save part of the batch for those units that are not compromised.
- Every rework procedure follows a set of instructions called a rework protocol. QA must approve all protocols before the production operator can execute them.
- Rework protocols are equally applied to in-process or entirely manufactured products, including those manufactured by the contractor sites.
- Before any rework begins, ensure all involved personnel understand the steps clearly. The production operator must perform line clearance, which ensures that the area is free from unrelated materials or documentation.
- Proper labeling and material verification are also necessary to prevent mix-ups and ensure full traceability of the reworked batch.
- Material reconciliation must be performed at the end of the rework, and the %yield should be determined. Reconciliation helps track the number of materials issued, used, and returned during rework.
- An acceptable yield percentage ensures that all materials are accounted for and helps identify any discrepancies.
- Rework protocol is considered part of the batch documentation. All rework activities must be documented in real time and signed off by the trained production operators before submission to QA for final assessment.
- Before a reworked batch can be released, QA has to provide final approval by reviewing all documentation, including the rework protocol, deviation reports, and quality control results.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
FDA requirements for rework procedure
The FDA 21 CFR part 211.115 outlines specific requirements for rework procedures in pharmaceutical manufacturing to ensure product quality meets GMP expectations. Key requirements include:
(a) Written procedures shall be established and followed prescribing a system for reprocessing batches that do not conform to standards or specifications and the steps to be taken to ensure that the reprocessed batches will conform with all established standards, specifications, and characteristics.
(b) Reprocessing shall not be performed without the review and approval of the quality control unit.
What is the rework procedure in ISO 13485?
ISO 13485:2016 – Medical Devices – Quality Management System outlines specific requirements for rework procedures. Key requirements include:
i. Documented procedures: Organizations must establish and maintain documented procedures for reworking nonconforming products. These procedures should detail the methods for rework, including retesting and re-evaluation, to ensure the product meets its original specifications.
ii. Evaluation of adverse effects: Before rework, a thorough evaluation must be conducted to determine any potential adverse effects the rework process may have on the product. This assessment ensures that rework does not compromise the device’s quality or safety.
iii. Retesting and re-evaluation: After rework, products must undergo retesting and re-evaluation to confirm they meet all applicable acceptance criteria and regulatory requirements. This step verifies that the reworked product conforms to its original design specifications.
iv. Documentation: Comprehensive records of rework activities must be maintained. Documentation should include details of the nonconformity, actions taken during rework, results of retesting and re-evaluation, and any determinations regarding potential adverse effects. These records are essential for traceability and regulatory compliance.
Rework procedure examples
Reworks are carried out mostly to correct defects in pharmaceutical products. They can occur on the bulk products production floor, packaging and labelling lines, laboratory testing, and warehouse picking and packing areas.
Example 1: Rework due to incorrect tablet coating
Situation
During routine in-process quality checks, you have identified that a batch of coated tablets had an uneven coating thickness due to a malfunction in spray parameters during the coating process.
You have raised a deviation. The quality assurance (QA) team assessed the product quality impact and determined that a rework procedure could be initiated that could correct the issue.
Action
The impacted batch of undercoated products are quarantined. Production Operator cleaned the coating machine, and a line clearance is completed.
After QA approved a rework protocol, the affected tablets were returned to the coating machine, and spray parameters were recalibrated to ensure even distribution.
This time, 100% in-process controls were implemented to monitor thickness consistency. The reworked batch was given a new batch number combining the original batch number with a Prefix (R -).
The sample of the reworked batch is sent for quality control tests, such as the tablet physical test, dissolution, and disintegration.
After the rework procedure, the tablets met all quality and regulatory specifications. The batch was successfully released for packaging and distribution. The process deviation was documented, and corrective actions (e.g., revised coating parameters, enhanced IPC checks) were implemented to prevent recurrence.
Example 2: Rework due to misaligned blister sealing
Situation
During packaging line inspections, operators noticed blister packs with faulty sealing, where some tablets were exposed due to improper alignment of the heat-sealing layer.
The line was stopped, and the impacted products were quarantined. The deviation investigation determined the root cause to be the misalignment between the aluminium foil roll and the blister pack where the filled tablets were placed.
The QA team suggested a partial rework was feasible as they could identify precisely the time the problem started and when it was discovered.
Action
QA approved a rework protocol where the tablets were carefully removed from the defective blisters and transferred to a new batch of compliant blister packs.
The sealing process was revalidated, and machine alignment was adjusted to ensure proper heat sealing. The reworked batch underwent leak testing and stability checks before final release.
The issue was successfully rectified, and the tablets were safely repackaged without contamination. The batch was approved for market release, minimizing financial loss.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Example 3: Rework due to incorrect batch labeling
Situation
A packaging batch of injectable vials was identified with an incorrect expiry date printed on the labels due to a printer calibration error.
After an investigation of the deviation, the QA team assessed whether the batch could be reworked efficiently, as the printer malfunctioned, avoiding a full rejection.
Action
QA halted the batch release and segregated the affected units.
A rework protocol was approved, allowing for the manual removal of incorrect labels under controlled conditions.
The batch was relabeled using the correct expiry date, ensuring full traceability. The final batch was subjected to 100% label verification before release.
Example 4: Rework due to out-of-specification tablet weight
Situation
During an in-process weight variation check, a batch of tablets was found to have inconsistent weights, exceeding the allowable tolerance range.
The production and QA teams conducted a root cause investigation and determined the weight variation was due to incorrect tablet granulation process. Malfunction of tablet compression machine was ruled out. Tablet weight machine was calibrated regularly.
Action
QA has segregated the batch, initiated a rework protocol, and concluded that the tablet batch could be reworked to meet the required weight without affecting the potency.
The affected tablets were re-blended with excipients and reprocessed through granulation and compression under controlled conditions.
The compression force was adjusted to ensure uniform tablet weight. A 100% online tablet weight verification was conducted on the reworked tablets.
As a result of the rework, the core tablets successfully met the required weight specifications, with all tablets falling within the acceptable range.
Example 5: Rework due to incorrect bottle capping
Situation
During the inspection of bottle filling operation, QA inspectors observed that a batch of liquid syrup bottles had loose or improperly sealed caps. This could impact the quality of the batch through external contamination, leakage and compromised stability.
The QA and production teams assessed through deviation investigation and determined that the deviation was found early and only a small portion of the batch was impacted. The capping issue could be corrected by reworking the capping process.
Action
The affected bottles were separated from the packing line. A rework protocol was approved. After the filling line clearance and quarantined batch was sent for rework.
The capping machine’s torque settings were adjusted. The line operators carefully removed the faulty caps manually and conducted a re-capping rework procedure.
The cap sealing integrity of the reworked batch was verified 100% through leak testing of the reworked bottles.
Torque measurements were recorded to ensure each cap was properly sealed to the required specification.
A sample of reworked bottles were put into stability trial testing and leak testing before batch approval.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Step-by-step instructions for rework procedure
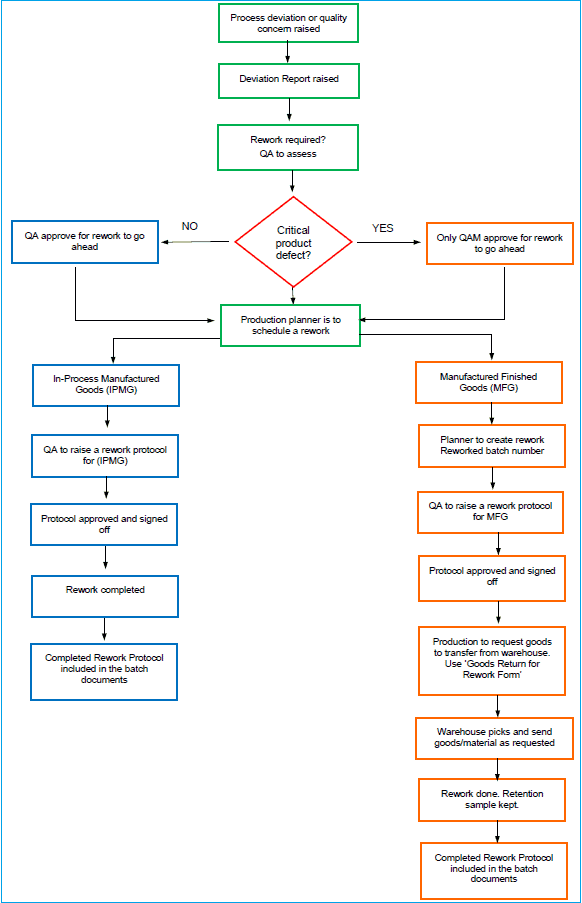
Follow the rework flowchart and the instructions carefully to execute the rework procedure effectively.
Rework of pharmaceutical products, in-process or finished goods, may be required for variety of reasons. Whatever the reason may be, the rework procedure if performed correctly should rectify the defects.
Step 1: Identify the deviation and CAPA
A product rework may follow as a corrective action from a deviation investigation. Hence, it is important to raise deviation as soon as quality defect is identified.
Ensure the deviation clearly details the nature of the problem and specifies the affected batch. Ensure deviation investigation is completed referencing the root cause analysis and recommended corrective actions supported by data and rationales.
Step 2: Quality assurance (QA) assessment
The QA team will assess the defect and determine the appropriate course of action. The QA Manager will review whether the batch can be reworked or if it must be rejected.
If the defect is critical and may affect product quality and patient safety, only the QA Manager authorises quarantining the batch and approving a rework. This decision must be documented on the batch record.
Step 3: Assign a reworked batch number
If a rework is approved for a fully manufactured product, the Production Planner must generate a reworked batch number to distinguish the reworked batch from the original.
Ensure the numbering format remains consistent but distinct from the original Batch number. This step allows clear traceability and prevents mix-ups.
For in-process manufactured products, a new reworked batch number is not required; the batch continues under its existing batch number.
Step 4: Develop and approve the rework protocol
Once rework approval is granted, the QA team must draft a rework protocol that outlines the corrective measures to be taken.
The protocol should include all necessary testing, reprocessing steps, and controls to ensure compliance with the original product specifications.
Before proceeding, obtain approval from the area manager and all relevant departments to confirm that the process aligns with standard operating procedures.
Step 5: Prepare for execution of rework procedure
The Production Operator must collect all necessary documents, including the approved rework protocol, visual displays, and batch documentation, to record the activities.
Contact the warehouse to verify the storage location of the affected batch. This information is typically found in the Goods Booking Slip, which records the storage type and bin location of the quarantined batch.
Step 6: Request goods for rework
The Production Operator completes the Goods Return for Rework Form or a Production Shop Order and sends the request to the Warehouse.
The Form or the Shop Order The form should have the following information:
– Person requesting the goods return, name, sign and date.
– WIP or designated area of the process line where the rework will be carried out
– Product Code
– Product Description
– Batch Production Number (BPN)
– Expiry Date
– Quantity to be returned
– Number of shippers
– Number of pallets
– Destination Storage type
– Destination Storage bin
– Shipper labels involved
The production operator has to sign the form and send it to the warehouse to arrange the transfer of goods.
The warehouse operator receives the form and picks up the requested stock from the source storage bin.
The warehouse operator must complete the warehouse section of the form before sending the goods to be reworked, including the source storage type and storage bins, the number of full shippers and partial shippers, and the number of pallets.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Step 7: Verification of materials to be used in rework
As the Warehouse delivers materials for rework, the Production Operator must verify the batch number, product, and other materials codes to confirm the correct materials have been provided at the requested quantities.
The Production Operator then applies labels identifying products to be reworked on all the pallets containing the affected goods. Proper labelling will ensure that products to be reworked are segregated from the original batch and handled appropriately.
If additional components or raw materials are required, the Operator must request the necessary items from the warehouse. This step is essential if reworking involves repackaging, blending, or reformulating the product.
Step 8: Perform the rework procedure
The Production Operator must perform a line clearance and ensure the rework area is clean and free of unrelated materials.
Ensure all Operators involved in the rework understand the procedures precisely.
Execute the rework according to the approved protocol, ensuring every action is performed under controlled conditions.
Document all changes and adjustments in real time to avoid any inconsistencies later.
If the rework involves correcting a quality defect, such as low tablet hardness, ensure that proper compression force settings are applied on the compression machine and that the outcome is monitored throughout the process.
Step 9: QA review and final approval
Upon completing the rework, submit the completed protocol for QA review. Before release, quarantine the reworked products in a designated warehouse area.
The QA team will inspect the reworked batch, collect samples, and perform necessary testing to confirm if the reworked products meet quality standards.
Attach the completed rework protocol along with other records, including the deviation report, line clearance checklist, goods picking slip, and original batch documentation with the reworked batch.
No reworked product can be released without QA approval.
Step 11: Clean-up and Final Documentation
After QA clearance, remove all temporary product-to-be-reworked labels and clean all surfaces using Isopropyl Alcohol (IPA).
Return the batch to the warehouse for final reintegration into inventory. Ensure that all documentation related to the rework, including the rework protocol, test results, QA approvals, etc., is securely archived for audit and compliance purposes.
Rework protocol for in-process and manufactured goods
A rework protocol is like a batch production record but only specific to the scope of the rework. Rework protocols are equally applied to both in-process and fully manufactured goods.
In-process manufactured goods are those that hasn’t completed the full processing operation. Defects are found and deviation raised in the middle of the processing.
For example, a batch of coated tablets with reduced thickness is an in-process manufactured goods.
Manufactured goods are those that have completed the full processing operation. Defects are reported after the goods are manufactured. For example, a batch of coated tablets released to further operation such as filling packing.
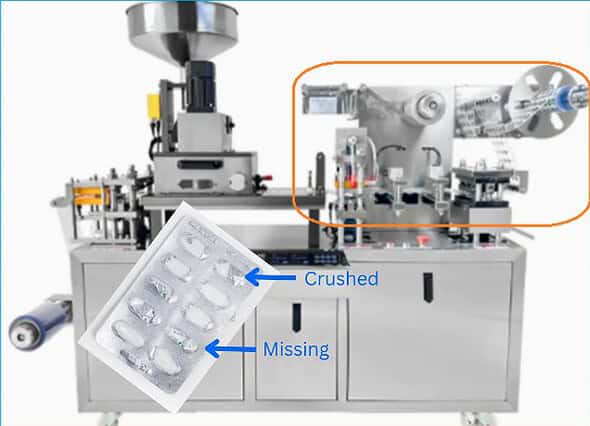
A rework protocol may be necessary for fully manufactured products due to defects identified such as printing error on the labels or crushed tablets due to misaligned blister foil during sealing process etc.
In the protocol you should include the necessary steps and instruction to carryout the rework process. This ensures that rework is conducted under controlled conditions and that the final product meets all predefined specifications.
i. Issuance of rework protocol
– After QA authorised the rework to be conducted a rework protocol is issued by the relevant department. The QA and personnel from the processing team must check all steps are correct and logical before the rework can be implemented.
– The protocol must clearly state the reason for the rework, whether due to a quality deviation, process failure, or other manufacturing anomalies.
– Look at the reason stated match with the root cause analysis and the proposed corrective actions in the deviation. Following are some of the examples that may be required:
Reprocessing: Conducting necessary adjustments in the processing operation such as blending, granulation, tablet compression, capsule refilling, or relabelling etc.
Repackaging: Removing packaging errors such as realigning foil with empty blister, correct expiry date on the labels or re-boxing as per specifications.
Re-inspection: Re-sampling in higher frequency and examining for defects such as leak testing, re-weighing, label inspection, looking for damaged packaging, or other quality issues.
– Include the following details in the protocol the product name, code, batch number, deviation number, expiry date, number of packs to be reworked.
– Ensure all operators involved in the rework process must sign off, confirming that they have read and understood the instructions outlined in the protocol.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
ii. Verification of batch details
– Cross-check the materials requirements are stated correctly. Such as the material description, batch number, quantity required, and quantity delivered.
– Report any discrepancies immediately to the responsible manager before starting the rework.
iii. Line clearance and rework execution
– Perform line clearance and ensure the rework area is clean and free from any unrelated materials.
– Isolate affected products from the processing line and ensure only conforming goods proceed through the rework process.
– Generate a goods return for rework form to request the warehouse to organise the necessary items to be sent to processing area.
– As the warehouse issue the materials identify and document the materials are received as required for rework.
– If necessary, request for additional materials if you identify there could be a shortfall in the middle of the rework.
– Follow a step-by-step process as outlined on the protocol to complete the rework.
– Ensure that all operators adhere strictly to the outlined instructions to maintain process integrity.
– Perform equipment checks before commencing the rework. Such as training the checkweigher, vision system, and label applicator etc if these are automated to ensure they function correctly.
– During the rework conduct in-process checks, including weight verification, seal integrity testing, and labelling accuracy checks.
– Maintain continuous documentation of every step in the protocol to ensure traceability and compliance.
– Operators must sign off at key points during rework to confirm adherence to the protocol.
iv. Material reconciliation
– After the rework, perform a material reconciliation of all materials used in the rework process. The reconciliation process includes:
a. Number of materials received (A)
b. Number of materials packed (B)
c. Number of materials sampled (C)
d. Number of materials rejected (D)
e. Number of materials returned (E)
Total material used (F) = (B + C + D + E)
– Calculate and record the yield percentage, using the formula below.
% Yield = (F/A) X 100
v. Final documentation and submission
– Ensure that all relevant documentation is completed, and the signed protocol is attached to the original batch documentation.
– Deliver the completed rework protocol and required sample pack as a retention sample to the laboratory for verification.
– All personnel must sign off, confirming completion of their assigned tasks.
vi. Quality assurance (QA) review and approval
– The QA department must review all documents, test results, and reconciliation records.
– If the rework meets the required quality standards, QA authorizes the release of the batch for further processing or market distribution.
– Any unresolved discrepancies or issues should be escalated to the QA Manager for final decision-making.
How to perform line clearance for rework procedure
Line clearance in pharmaceutical industry is a set of activities or checks performed just before an operation can commence.
The line clearance procedure ensures the processing line is free of any irrelevant products, components, and documentation that could be left accidentally from an earlier batch and may cause mix-ups or contamination.
Line clearance is the first step in a process, and it is to be done before a line opening.
Line opening ensures correct products, components, and documentation are brought into the processing line so the operation can commence without error.
You can commence the rework procedure after a successful line clearance, setup, and opening.
The final step in the sequence is line cleaning, which ensures no raw material, components, or processing records are left behind on the line to avoid mix-ups with the next lot.
Follow the steps below to complete a line clearance.
– Place a “Line Clearance in Progress” sign in a visible area before beginning the process.
– Check that no previous product, labels, or components remain on the line. Remove any residual materials.
– Ensure that all unused materials from the previous batch have been cleared.
– Fill out the line clearance checklist step by step. The checklist must be signed off by two authorized persons for additional assurance.
– If the checklist is partially completed and a new operator takes over, the entire procedure must be redone.
– Document any discrepancies must on the non-compliance logbook, report to the area manager, and addressed before production begins.
– Include the line clearance checklist in the final batch documentation which can only be signed off after a successful line clearance is completed.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How to calculate yield percentage during rework procedure
Yield percentage is the outcome of good reconciliation and a key metric during rework procedure. A good % Yield ensures all issued materials are accounted for during the rework process.
Use the following formula when you calculate % Yield:
% yield = | (No. of goods produced + Rejects + Samples + Returned) | X 100 |
(No. of goods received at the start of process) |
Key points for recording yield percentage:
– You should perform the reconciliation in real time immediately after each step to avoid discrepancies.
– Include the count of all materials and components used in the rework, including the quantity of finished products subjected to rework, products produced, rejected items, samples, and returns.
– To be practicable, use an allowable variation (e.g., 99.00-101.00% for labels, cartons, and leaflets) to account for minor inconsistencies.
– If the yield falls outside acceptable limits, conduct a recount. If discrepancies remain, raise a new deviation for further investigation.
– Remember, errors can result from incorrect counts, material mix-ups, or losses during rework.
Rework procedure in contractor facility
Pharmaceutical companies often work with contract manufacturers for production and packaging.
When the contract manufacturer reports a quality defect, the rework procedure must be handled carefully in coordination with the manufacturer before the finished products are accepted.
Rework must be done while ensuring compliance with contractual agreements and quality standards.
The goal is to correct defects without compromising safety, effectiveness, or regulatory compliance.
Step 1: Justify the need for rework
When a quality defect is found at the contractor site, the QA team must evaluate whether rework is possible. If needed, meet the contract manufacturer to discuss the issue and determine the best course of action.
Step 2: Check the quality assurance agreement
Before the rework procedure is carried out, ensure a quality assurance agreement permits such activity and includes both parties’ responsibilities.
Create a third-party manufacturer dispatch report to track product movement. Then, raise a Purchase Order to authorize the corrections.
Step 3: Develop a rework plan
The QA team must create a detailed rework protocol. This document should outline:
– The reason for rework
– The related deviation report number
– Specific corrections needed (e.g., processing, packaging, or testing etc.)
– Acceptance and rejection criteria
– Material reconciliation and final product yield
Before the rework starts, this protocol must be reviewed and approved by both the Contract Giver and the Contract Manufacturer.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Step 4: Review contractor’s work instructions
The contractor must prepare work instructions based on the approved rework protocol.
The Contract Giver’s QA team must review and approve these instructions to ensure they follow cGMP requirements.
Instructions should include equipment settings, worker training, and product handling steps to avoid contamination or further errors.
Step 5: Ensure material availability and logistics
The procurement team must confirm that all required materials, such as packaging, raw materials, or laboratory reagents, are available for rework.
If more materials are needed, a material transfer order must be processed between the sites. The supply chain team must also arrange the transport of defective goods to and from the contractor facility.
Step 6: Execute rework under supervision
Once the protocol is approved and materials are in place, the contractor can begin rework following the approved protocol.
Trained personnel at the contractor site must follow the instructions provided in the rework protocol and document each step in real time.
The contract giver’s QA team may conduct on-site inspections or virtual audits to ensure the rework is conducted under a controlled environment. In case new issues arise, the contractor must notify the contract giver immediately for resolution.
Step 7: Perform quality control and testing
After rework, the contractor must conduct all necessary quality control tests as required by the protocol and the agreement to ensure the product meets specifications.
If required, the Contract Giver’s QA team reviews test results and may conduct further verification.
The Contract Giver’s QA team should assess if additional stability testing is required.
Step 8: Approve and release reworked products
If the reworked product meets all specifications, the Contract Giver’s QA team issues final approval for batch release.
The contractor’s warehouse team returns the material to inventory and prepares it for shipment.
The Contract Giver must ensure that all documentation, including the third-party manufacture dispatch report and certificate of analysis (CoA), is received before integrating the reworked batch into the supply chain.
Conclusion
Rework is common in pharmaceutical manufacturing. A well-coordinated rework procedure ensures that defective products are corrected while maintaining quality, safety, and compliance with regulatory standards.
A well-structured rework can prevent unnecessary waste and allow companies to recover part of the batches that might otherwise be discarded.
All rework activities start with a carefully crafted rework protocol. A well-designed protocol ensures that defects are addressed without compromising product integrity. Whether rework occurs in production, packaging, or contract manufacturing, it must be controlled, supervised, and recorded in real time to meet industry standards.
During the rework, every step, from identifying deviations to final QA approval, must be carefully recorded in the batch documents to maintain traceability and compliance.
Ultimately, a well-managed rework process helps pharmaceutical companies maintain product quality, reduce financial losses, and ensure that only safe and effective products reach patients.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.