
GMP rules to keep pharmaceutical warehouse in perfect condition
- Published on: Apr 03, 2024
Pharmaceutical warehouses play a pivotal role in the manufacturing of quality products.
The quality of a pharmaceutical product is defined by its purity, efficacy, correctly identifiable characteristics, and safety of use. Customers and patients have an ethical (and legal) right to expect pharmaceutical products to be of the highest quality.
Good warehousing practices ensure just that. Warehouse activities help ensure products remain safe, pure, and effective while stored.
A pharmaceutical warehouse is responsible for receiving, storing, and releasing incoming goods (including labeling and packaging) and distributing finished products.
Therefore, GMP rules ensure that materials are handled and stored properly and that appropriate documentation is maintained.
GWP vs. GDP
Good Warehouse Practice (GWP) refers to practices within the company warehouse.
Good Distribution Practice (GDP), on the other hand, refers specifically to the transport and distribution of the product.
– GDP and GWP are each special parts of GMP.
– GDP and GWP each have their legal definitions and regulations. These regulations recognize that product quality can be significantly impacted if manufacturing and packaging have taken place.
– In this module, “GMP” for the warehouse incorporates practices, rules, and regulations spanning GMP, GDP, and GWP.
Once the pharmaceutical warehouse receives a finished product, it does not undergo any further inspections or quality control tests.
If the product is degraded or damaged at this point, nothing stops it from being given to the patient.
The warehouse must rely upon procedures and well-trained staff to ensure that products arrive safely and with the same quality as when they left manufacturing.
Many products have been affected by poor warehouse storage conditions or rough handling during transport.
Biopharmaceutical products often have temperature-sensitive active ingredients that break down or degrade if exposed to heat or light, thus becoming ineffective.
A pharmaceutical warehouse must be expertly managed and run in compliance for the company to protect and distribute a quality product.
These compliant practices include control over receiving goods, quality control, storing materials, components, and products, fulfilling picking requests, and shipping the product to the marketplace.
These practices must be completely traceable to protect the integrity and stability of the product and its packaging.
GMP rules for the warehouse enable manufacturers to:
– Protect medicines from damage during storage and transport.
– Prevent degradation of the product by exposure to adverse temperature conditions.
– Avoid mix-ups and contamination by other materials.
– Maintain product identity and traceability.
– Prevent time-expired or damaged material or product from being used.
What comes into the pharmaceutical warehouse?
The following goods generally do not appear on any production bill of materials and have a simplified check and release.
Often, they do not have an in-house lot number applied, but this varies from company to company.
– Non-production consumables (non-GMP material), e.g., toilet paper, stationery
– Production materials consumed in processing, e.g., filters, lubricants
– Laboratory reagents, e.g., buffers, chemicals
The last two items above will usually have their own QC approval processes.
The following goods will always appear on any production or packaging bill of materials.
They are each governed by GMP quality control and release procedures.
All these goods will be issued with unique lot numbers.
– Manufacturing starting materials and chemicals
– Packaging components, e.g., blister pack film, bottles, caps, vials, seals
– Printed matter, e.g., labels, cartons, inserts/leaflets, pre-printed tubes
Procedure for receiving materials in the warehouse
When starting materials first arrive at the facility, they fall under the control of the warehouse.
Different types of materials can be treated in various ways.
For example, materials consumed in production need to be registered and checked for approved supplier and grade (pharmaceutical or medical).
Still, they may or may not need a unique identifying number.
Please make sure that you know your Standard Operating Procedures.
In the case of starting chemicals used in product formulation, they will always need to be checked to see that they;
– Arrived from an approved supplier
– Are not damaged
– Are correctly labeled and identified with the supplier’s lot number for traceability purposes
– Are given a unique in-house lot number
– Registered in inventory; and
– Quarantined, then sampled for release testing
Some of these chemicals may also deteriorate when exposed to heat, so they may have to be stored under controlled temperatures or frozen.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
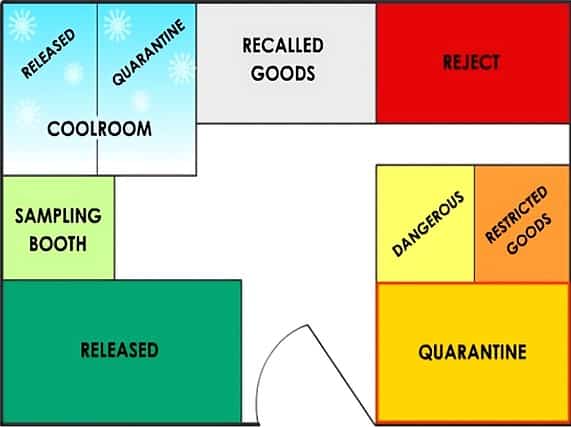
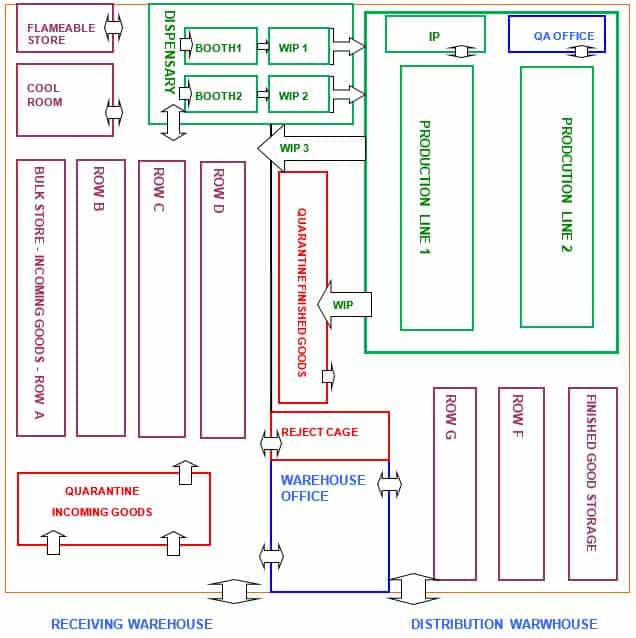
Typical layout of a pharmaceutical warehouse
Pharmaceutical warehouses are typically classified by the types of material they contain, such as raw materials, packaging materials, intermediate or bulk products, and finished products.
A typical warehouse will contain some or all of the areas described below.
– Quarantine area for storing goods that have not been inspected or tested. Materials stored in Quarantine cannot be used or released until approved by QC.
– Some warehouses have a Dangerous Goods storage area to ensure the safety of staff and the facility. For example, flammable goods such as ethanol would be stored in this area. Special storage conditions, such as flame-proof cabinets, are used here.
– Some warehouses have a locked area for restricted goods, such as poisons and drugs of addiction. This area is restricted to specifically authorized staff.
– A separate area for isolating faulty or recalled goods, ensuring that they are not issued or sold by mistake.
– A “reject” area reserved for rejected batches.
– The cool room is usually operated at 2°C – 8°C so temperature-sensitive materials (e.g., vitamins) do not deteriorate.
You must regularly check that the cool room temperature is within normal operating ranges. The cool room usually has a quarantine and a released area.
The sampling booth is the first place where chemicals are opened.
To protect the material, the sampling room should have specific air controls to exclude outside air from entering and to contain any dust generated from sampling to protect the environment.
Sampling implements must also be cleaned before use and cleaned after each use.
Pay particular attention to the air control systems, gowning, and safe handling of chemicals in this area.
– A “released” area for batches successfully tested and passed for use.
– A quarantine area for storing goods that have not been inspected or tested yet. Materials stored in Quarantine cannot be used or released until approved by QC.
Try our FREE online GMP Skill Booster tests. It’s challanging, it’s refreshing and it’s FREE. Try now!
Use of computer systems in the warehouse
Computer systems often control the inspection and test status of materials and products.
Materials and products may not need physical status labels or be stored in separate areas if computers are used.
Computer systems used in warehouse inventory status control must be validated for reliability and free from errors.
It would be best if you established strict procedures and security levels to ensure that information is accurately entered and be familiar with the security and procedures associated with using these systems.
Repacking in a pharmaceutical warehouse
Some warehouses in pharmaceutical manufacturing may decide to repack larger containers of raw materials into smaller order quantities.
While this may seem simple, it must be performed under GMP controls and accompanied by a processing record.
Repacking should be performed according to a written procedure and only by trained staff.
Common mistakes made during the repacking process?
– The smaller bags’ labels may give different information than the original larger pack.
– The repacking may occur in a room contaminated with other chemicals.
– The smaller containers may not be compatible with the giant drum.
– The re-packer may want to apply their company name and delete the original manufacturer’s name.
– The chemical may be hydroscopic (pick up moisture) during repacking.
– The chemical may be harmful to health if not handled correctly, or the Material Safety Data Sheet (MSDS) may not be included.
Warehouse personnel and training
Warehouse personnel in pharmaceuticals need to be skilled in several different functions, including applying the GMP rules specific to the warehouse, just like production operation personnel are trained in using the GMP rules related to their areas.
All warehouse personnel must be trained. Even the contract and casual staff employed during peak times must have adequate training on good warehousing practices.
Warehouse personnel must know the standard operating procedures applicable to the warehouse’s operation.
Following are some examples of specific skills the warehouse personnel should be trained in:
– Pack and label stock so that it is protected during transport.
– Interpret environmental monitoring displays such as temperature and humidity gauges.
– Be able to ensure materials and stocks are stored in their correct storage locations.
– Use safe handling procedures according to each chemical’s Material Safety Data Sheet (MSDS).
– Conduct stock takes and maintain inventory records.
– Accurately locate and select the right stock from pick lists and orders on a first-fin, first-out basis.
– Recognize situations where products or chemicals may be at risk, e.g., spillage, broken pack, missing labels.
– Picking machinery, such as a forklift or bay picker, should be safely operated.
– Accurately enter stock into the computer system inventory and maintain accurate paper records.
Preventing contamination and deterioration in the warehouse
To prevent possible contamination or deterioration of materials during warehousing, some essential rules must be followed:
– No packs should be left unsealed. This prevents possible dirt contamination from the environment or pest infestation.
– All chemicals and products must be stored within their labeled temperature zones; for example, some product labels will require you to store the following:
i. Store at 2°C-8°C.
ii. Do not freeze.
iii. Store below 25°C.
– Use a first-in, first-out (FIFO) system. Chemicals and medicinal products deteriorate over time and generally have expiry dates. Not rotating stock could result in partly degraded or aged materials being issued.
– Follow the chemical and biological spill response procedure to protect your staff and products from unintended contaminations.
– Please report all incidents to the Supervisor as soon as possible.
– Keep outer doors and exit doors closed whenever not in use. Dirt and pests from outside may contaminate the stock.
– Never use material or product that is either unlabeled or not correctly labeled. If a label is missing, it generally means the stock should not be used.
Preventing mix-ups in pharmaceutical warehouse
In a busy pharmaceutical warehouse, starting materials are transferred daily in and out of the warehouse.
Pickups from production, storage of in-process and bulk goods, management of incoming finished products, and release of finished stock to distributors are common.
Space is often limited, and many warehouses can become overcrowded.
During all these stock movements, staff have to maintain records and sometimes apply labels to indicate status.
As there are opportunities for mix-ups to occur on an almost daily basis, so too are there GWP rules that help prevent these from happening:
– Quarantine inward goods and finished goods until they are approved for release.
– Segregate material by their status labels (e.g., only released labeled stock should be in the released store).
– Ensure that Unique Identifying Numbers and batch numbers are visible and legible.
– Wherever possible, ensure that only one type of material is stored in one location. (Note: Different companies may have different views on this)
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Why do materials and products need segregation?
Raw materials and finished products must be physically segregated in pharmaceutical warehouses unless they are managed through validated electronic inventory controls.
One main reason for segregation is to ensure that only the correct materials or products are released.
In the case of defective and recalled goods, it is essential to have both physical and electronic segregation.
Physical segregation
It is a GMP/GWP rule that goods with different statuses be physically located in different and designated areas if a validated computer system is not used to control location. (Some companies also use physical segregation even when they employ validated computer systems)
Physical segregation minimizes the chance of someone accidentally selecting and using the wrong material.
Could you always double-check the item’s status and location before selecting it?
If the selected lot is in a quarantine or reject location, could you refer the error to your supervisor?
Electronic segregation
Many warehouses control their inventory using an MRP computer system. The system must be validated (100% reliable) and only allow access to authorized personnel.
Generally, computer systems in pharmaceutical warehouses are used to:
– Register inward goods and their quantities, lot numbers, expiry dates, and suppliers, and assign their status (e.g., Quarantine)
– Change the status of a lot or batch
– Depending on the status of the lot or batch, allow or disallow planners and dispatchers from selecting goods electronically
– Track inventory movement to specific allocated locations in the warehouse so that only the right material or product can be selected for use or release
– Provide an accurate history of stock movement to allow for stock takes and traceability
In summary, the warehouse computer system serves a critical function, and the staff required to use it must be fully trained.
In addition, staff should never share their passwords with other staff nor log on or off other personnel.
Warehouse personnel have to deal with materials and products that may have different statuses or may change status during storage.
Some materials and products may be damaged or defective, and staff need to isolate these from good stock.
The following non-routine, suspect, or defective goods may be encountered in the warehouse:
– Damaged goods from stock
– Returned goods from customers
– Recalled goods
– Counterfeit products
– “Not for Sale” samples
GMP rules for pharmaceutical warehouse
A fundamental GMP control ensures that only approved starting materials that meet specifications are used to formulate medicines. Patient safety depends on it.
Removing the protection of status controls will raise the risk of an error.
Government regulatory agencies and their auditors are very aware that a lack of control over material movement in the warehouse can and has led to defective products.
For this reason, they regularly inspect the warehouse and its procedures to ensure that GMP rules are in place.
1. Rules for contamination
Contamination refers to the presence of any foreign substance in a product. It may be:
– Physical, e.g., foreign objects, dirt, dust, pollens
– Chemical, e.g., cleaning agents, lubricants, impurities, degradation products
– Microbiological, e.g., bacteria, molds, spores, yeasts
The main focus of GMP in the warehouse is preventing contamination and mix-ups of materials and products.
Some general rules to prevent contamination and mix-ups are:
– Materials must be purchased only from approved suppliers to written specifications.
– All incoming materials must be placed into quarantine.
– Quarantined materials must be inspected and tested to verify their suitability.
– All materials must be labeled to indicate their inspection and test status.
– Materials must be stored safely to protect them from deterioration and damage.
– Records must be maintained for all issued material to ensure traceability.
2. Material identification information
Each material received should be labeled with a standard name and a Unique Identifying Number (UIN).
These are used throughout storage and processing to identify that material.
To avoid confusion with product batches, the UIN is generally not called a batch number and should differ from the supplier’s lot number.
i. Supplier’s batch number
Containers bearing different manufacturers’ batches or lot numbers should be separated within each delivery from the supplier.
This will help trace any contamination or other problem to a supplier’s batch. Thus, it is easier to isolate finished products suspected to contain foreign material.
Each group bearing the same batch, lot, or equivalent number in a single delivery can be regarded as the same material.
Subsequent deliveries of the same manufacturer’s batch or lot number should also be regarded as separate materials since the handling and transport may have affected their quality.
ii. Standard name
The standard name refers to the unique approved name listed in the company’s standard names register.
The standard name is the same one that is used in the product formulation (bill of materials).
For example, sodium chloride can also be generically called salt or sodium salt. Standardizing the name internally eliminates any confusion.
The term “USP” refers to the grade or quality of the material. Generally, chemicals will be labeled “USP,” “British Pharmacopoeia,” or “EP” grade to indicate that they are pharmaceutical grade.
iii. Material item code
The material item code is linked to the Standard Name and is used in the Master Batch Record and formulation.
This code also corresponds with the barcode and usually helps identify the approved supplier and grade of the material.
iv. UIN (Unique Identifying Number)
The UIN (Unique Identifying Number) is alternately called the Goods Inward Number (GIN), lot number, or batch number.
The UIN is critical because it uniquely identifies and allows traceability of a consignment of material. When a product is formulated, the UIN is recorded in the laboratory, on the computer system, and in production.
v. Expiry date
The expiry date indicates when the goods cannot be guaranteed safe for use. Once they have expired, they must be placed in reject areas and not used.
vi. Storage conditions
Materials and products can degrade if not stored under the approved storage conditions.
For finished products, storage conditions are registered with the government, making it a legal requirement to store them in compliance.
vii. Barcode
Some companies apply a unique barcode, providing a second unique identifier for the material and product.
The barcode is used to scan the identity of the item before it is issued electronically.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
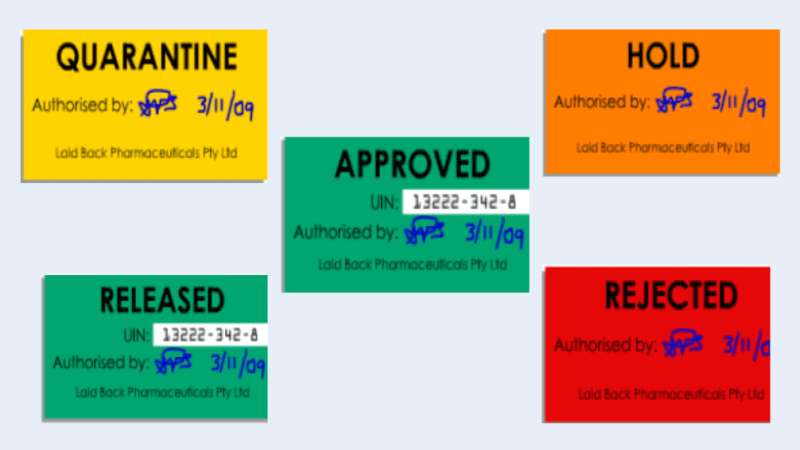
3. Status labels used in pharmaceutical warehouse
In addition to the identification information, each lot requires a status label to indicate the status of any material.
Some examples of physical status labels are below. Note the use of the different colors. (Note: Different companies may have different colors for the types of status labels used)
Status labels may be either:
– Physical labels on the container
– Electronic fields on a database
– Combination of physical and electronic
Please be familiar with your company’s system to make sure that only released materials are used.
Before any lot may be used in manufacturing, it must be released for use by quality control (QC).
Once a lot has passed inspection and testing, QC will change the status from “Hold”/”Quarantine” to “Released”/”Approved”.
i. Quarantine label
This label is used when the goods have yet to be tested and released for use by the laboratory. Materials labeled Quarantine should not be used.
ii. Hold label
The Hold label indicates the material may have a problem and is under investigation. Please do not use it under any circumstances.
iii. Release label
The Released label indicates that the material is suitable for use or has been released for supply. The batch has been tested and cleared by the quality department.
iv. Approve label
The approved label indicates the material is suitable for use or has been released for supply. The batch has been tested and cleared by the laboratory.
v. Reject label
The Rejected label indicates that the batch has been assessed as unacceptable or defective. Never use these materials. They should be located in a separate, locked area.
4. Control of product labels
Controlling product labels is crucial to GMP since mislabeled products may adversely affect customers.
All product labels must, therefore, be strictly controlled. Some of these controls include:
– Each different type of label is identified and segregated in the label store to prevent mix-ups.
– Access to the label store must be restricted to authorized personnel.
– There should be no loose approved or released labels in the store.
– Any unused or damaged labels should be returned to the Quality Department.
– “Approved” or “Released” labels should only be issued by the Quality Department.
– Before the release of labels, they must be checked and double-checked against the work order to verify the following:
i. Product name and code
ii. Label identity and version number
iii. Coded batch number and expiry date
Materials can be taken into the packaging area only when all the checks have been satisfactorily completed.
5. Storage requirements for pharmaceutical warehouse
Starting materials and components must be stored in designated locations.
Some materials are required to have additional safety and security procedures. For example, the following goods must be locked up or otherwise secured from unauthorized access:
– Printed packaging materials.
– Drugs of addiction, antibiotics, and highly potent drugs.
– Poisons and flammable goods.
– Rejected and recalled materials.
– Returned goods.
Keep powders, liquids, and corrosive and flammable materials separate.
Dangerous goods areas should be specially equipped to prevent contamination and spills.
How to keep the warehouse Clean?
GMP rules require that warehouses storing raw materials, components, printed matter, and finished goods be always clean, dry, and orderly.
This involves, but is not limited to:
– Protecting receiving areas from the weather.
– Checking regularly to ensure that containers and packs are sealed properly
– Cleaning the facility regularly
– Cleaning up spills immediately
The exterior of inward goods containers may also need to be cleaned before storage.
i. Protecting goods from deterioration
To protect goods from deterioration:
– Store goods at the temperatures specified on their labels.
– Monitor and alarm freezers and refrigerators.
– Monitor temperatures in other areas where goods requiring specific storage conditions are stored.
– Review all records; analyze and file as appropriate.
– Investigate all deviations and assess their impact on product suitability.
ii. Protecting raw materials in a warehouse
Because raw materials may deteriorate on storage or become contaminated if stored incorrectly, the storage conditions are critical to product purity.
To protect raw materials, there are some specific and important GMP rules relating to this:
– Separate quarantine and reject areas.
– The storage environment should be temperature-controlled.
– Store goods off the floor and away from walls.
– Employ a system that identifies material about to expire so that it can be retested if necessary.
– Periodically inspect stockrooms for past-expiry or expired stock, cleanliness, housekeeping, and pest control.
– Seal over all opened containers to protect them from the environment.
– Ensure a cleaning program for each separate area in the warehouse, including external areas, gangways, shelving, and racking.
Try our FREE online GMP Skill Booster tests. It’s challanging, it’s refreshing and it’s FREE. Try now!
iii. Pest control
Pests attracted to pharmaceutical warehouses include rats, mice, birds, ants, and cockroaches.
Stored goods in the warehouse can be contaminated by bird or rodent droppings.
Therefore, GMP rules require that a manufacturing facility has:
– A nominated pest control officer.
– Specific instructions or agreements with pest control companies.
– Maps showing bait locations.
– Documentation of all pest control treatments.
– Having frequent waste collection.
Environmental regulations and OH&S also require companies to protect personnel and the surrounding environment from the chemicals used in pest control programs.
An effective pest control program involves:
– Keeping track of any sightings of pests between visits by the pest control companies
– Assigning someone to accompany the pest control agent when they are on-site
– Using only approved pesticides
Controlling air supply in the warehouse
The warehouse links the outside environment to the inside production facility.
Therefore, the warehouse becomes a barrier to prevent dirt, dust, particles, moisture, and pests from entering the building.
In order to separate the inner production area from the outside, warehouses generally have specific designs and rely upon employees keeping doors shut wherever possible.
i. Outside air
Of course, the outside air is not controlled in any way.
If the factory is left open, the wind will blow contamination into it, so the outer door must be kept shut when not in use.
The outer door provides the first point of protection for the warehouse and the production area.
ii. Receipt area
This is where goods are unloaded off the delivery truck and assessed before being brought into the warehouse.
Notice that this area has an outer roller door and an inner curtain to prevent outside dirt or dust from entering the warehouse.
An important GMP rule is not to have both the outer roller door and the inner barrier open simultaneously.
This area is sometimes referred to as a “black” zone.
This area is also the first point where outer bags and cartons are cleaned so that minimal dirt is transferred to the warehouse.
If goods are unclean or damaged, they should not be sent to the warehouse.
iii. Warehouse area
The warehouse itself is not pressure-controlled but is cleaner than the receipt area.
In the warehouse, goods should be stored off the floors and away from walls because moisture may be taken up through concrete walls and floors.
This area is sometimes called a “grey” zone since it is cleaner than outside but still not clean enough to expose products to the environment.
iv. Production area
Some companies call the production area a “white” or “clean” zone, where only cleaned materials may be taken.
Generally, goods transported to this area are on plastic pallets since wooden pallets cannot be cleaned and can harbor fungus, mold, and bacterial contamination.
The pressure-controlled production zone provides an air barrier to entry into dirt and particles.
To maintain positive pressure, it is critical to keep the doors closed between the production and warehouse areas.
Before materials are taken into the production area, the outer cartons that had arrived on the truck are generally removed, where possible, as an added precaution.
Most companies also have different dress codes when moving from a grey zone to a white zone.
Importance of controlling storage temperature
Temperature storage controls are important in pharmaceutical warehouse in order to ensure product integrity throughout the product’s shelf life.
Medicinal products damaged by exposure to extremes of temperature may escape detection, resulting in physical damage and loss of therapeutic effect. Worst of all, it may not be obvious at all.
Companies are expected to create a temperature map of temperature-controlled warehouses.
The map is used to identify the best places in the warehouse to store goods.
When creating this map, an initial study will reveal where problems would most likely be encountered, e.g., storing goods against north-facing walls, close to room heaters, windows, or the roof.
After initial mapping, temperatures are routinely monitored continuously or daily.
This monitoring may reveal seasonal patterns where temperatures rise or fall outside approved limits.
Therefore, it is important to continue to review temperature profiles in the warehouse over time, particularly during summer and winter.
Some companies monitor using an electronic building management system.
i. Ambient temperature storage
The terms “room” or “ambient” temperature storage conditions have different meanings to different companies and different regulators.
It is now more common to use the term “temperate” storage. Temperate storage has recently been redefined as a storage temperature of between 15°C and 30°C, with occasional excursions above that range.
Temperate storage controls vary from product to product. However, many licensed products stipulate an upper limit of 25°C with no lower limit apart from “protect from freezing.”
Inspectors have indicated that 8°C to 15°C is acceptable for temperate storage.
ii. Additional requirements for temperature control
– The storage conditions for the goods should be compatible with the storage conditions specified on their labels.
– Controlled storage environments should be monitored using suitable temperature recording devices.
– All records should be reviewed and filed, with their results tabulated and analyzed.
– Refrigerated and freezing storage environments should be fitted with both an alarm and a visual signal. This signal should permit resetting only by an authorized person.
– If any temperature is found to have deviated outside the relevant recommended conditions for an extended time, the manufacturer of the goods should be contacted, and the product’s suitability for use should be resolved.
– Equipment used for monitoring temperature should be calibrated regularly to ensure accuracy.
iii. How to create a temperature map?
A simple temperature control example involves the physical location of material storage in a pharmaceutical warehouse.
The higher up in the warehouse a material is stored, the hotter the temperature the goods will be exposed to. So a physical space nearer the ceiling without temperature controls will be inappropriate for storing goods that need to be kept cool.
– Study the temperature profile of the premises and identify potential “hot” and “cold” areas.
– Study the areas of greatest potential risk using calibrated temperature and humidity monitoring instruments.
– Develop a temperature mapping validation protocol.
– Based on initial findings, could you start monitoring regularly?
– Produce a temperature map of the premises.
– Review the hot and cold areas when outside temperatures are extreme, for example, in summer and winter.
– Review the controls of heating and cooling systems.
– Ensure an action plan exists in the event temperatures become compromised.
iv. Cold storage
Cold storage refers to the storage of products requiring a storage temperature of 2°C-8°C or when the material is required to be stored frozen (e.g., below -20°C or below -70°C).
For a successful cold storage operation, the facility must select the right temperature recording instrumentation, validate its processes, and maintain warehouse documentation of records and procedures, including responding to alarm conditions.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Validation and documentation of temperature control
Validation efforts must:
– Prove that temperature mapping checks for areas of extreme temperatures.
– Check on temperature variations during use.
– Demonstrate performance in the event of a power failure.
– Demonstrate the function of alarms.
Specific procedures that need to be incorporated include:
– Check temperature records at the start of the waking day and record these checks in a log.
– What do you do when certain staff are absent?
– Responding to alarm conditions.
When reviewing the cold storage controls, a program should be set up to review cold store temperature records weekly and monthly as part of a self-inspection program.
Instruments used in pharmaceutical warehouse
The selection of recording instruments is vital.
The instruments must be easily read, and the data must be retrievable.
They must also be calibrated to a national standard (the company should retain a copy of the calibration certificate for the equipment) and incorporate a high/low-temperature alarm. Max/min thermometers are usually not acceptable.
Both air and product temperatures should be recorded. Note that air temperature can fluctuate considerably more than product (often called “load”) temperature.
i. Monitoring alarms
Out-of-hours alarm signals are often sent to remote contractors or security offsite.
It’s, therefore, very important to ensure the alarm system operates reliably, as repeated false signals may result in a real signal being ignored.
There are many industry examples of degraded stock due to late or no response to warehouse electrical failures. The alarm system should operate independently of the main electrical system.
There must be a written procedure on how to respond and who to inform when a temperature alarm monitor signals a problem.
This procedure should cover initial action (e.g. quarantining the cold store) and subsequent action.
There should be an available and current list of emergency numbers to call, and there should be a backup plan to relocate any products are risk of deterioration (e.g. frozen stock).
Any response must be immediate, so the procedure should ensure that weekends are addressed as well.
ii. Protecting sensitive materials
Many companies (particularly those manufacturing biologicals and vaccines) handle, store, and transport temperature-sensitive raw materials and finished products.
Protecting these products is critical to their effectiveness and safety.
Products can be degraded by excessive heat, moisture, light, or repeated freeze/thawing cycles.
iii. Freezers
Frozen materials are usually stable at -20°C and below, though some require freezing at -80°C.
If the temperature rises, it will probably do so slowly over several hours.
Once the temperature rises above a certain point, some frozen materials will start to deteriorate or degrade.
A written action plan must include a contingency for relocation if the temperature continues to rise.
iv. Automatic defrost refrigerators
Some cold storage refrigerators have an automatic nightly defrost cycle. These refrigerators may not be suitable, as many frozen products can partially melt and re-freeze, causing them to deteriorate and degrade.
v. Heat and moisture
Almost all medicinal products are sensitive to heat and will degrade at higher temperatures.
Some products will degrade at room temperature, while others can withstand temperatures above 30°C for prolonged periods.
Knowing which products are at risk or degrading at which temperatures is practically impossible.
All products must undergo stability studies to prove they will not degrade under the approved storage conditions.
GMP rules require that products be stored only within approved temperature ranges. A deviation notice should be raised if a product is exposed to excessive heat.
Some products may absorb moisture from the atmosphere if not properly sealed.
One of the main causes of moisture problems is incorrectly storing products directly on concrete floors and walls, as the product packaging can absorb moisture from these surfaces.
vi. Light
Some products are “photolytic”; that is, they may degrade if they are exposed to light for prolonged periods.
Be alert to packages that state “Protect from light” and ensure they are completely sealed.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Housekeeping audits in pharmaceutical warehouse
One of the prerequisites of a perfectly maintained pharmaceutical warehouse is to conduct regular housekeeping audits.
When I was a GMP Auditor, my key focus of any site audit was to ensure the health of the warehouse.
I observed that warehouses are best maintained when housekeeping audits are performed weekly and monthly.
You can take a checklist approach for warehouse audits. Together with the warehouse and QA staff, prepare a comprehensive set of questionnaires covering all areas of warehouse activities.
Prepare a schedule for self-audit and conduct the audits as scheduled. Monitor non-conformances and implement effective corrective actions.
Please be sure to look for any adverse trend that is forming and address those concerns before the problem grows too big to control.
A typical warehouse audit checklist should include the following areas of concern:
i. Warehouse office areas
– Is documentation appropriately stored?
– Contact lists near phones are clearly displayed, current, and easily accessible.
– All personal items are stored in lockers.
ii. Management of standard operating procedure (SOP)
– Are SOP files accessible and filed alphanumerically?
– Have the superseded versions been returned to QA?
iii. Environmental, health and safety
Would you happen to know if all fire/emergency exits and stairs are clear?
– Safety signs are clearly visible
– Access paths are unimpeded
– Forklifts parked away from traffic flow with tines lowered and keys removed when unused.
– Fire extinguishers/hoses are visible and accessible.
– All fire extinguishers are within the calibration range.
– Floors are clear of spills, chemical residue, and excess materials.
– Emergency stop buttons are visible and accessible.
– No bin sheets on items
– The heaviest items are stored on lower shelves.
– All cardboard was removed, except where necessary for goods storage.
– Cardboard should be kept at a minimum level.
– Clear access to shelving
– Safety equipment worn and used for appropriate tasks (ear protection/safety glasses/gloves)
iv. Staging and shrouded areas
– Area waste bins are not overflowing.
– Waste bins are used for appropriate materials only.
– Empty pallets are stored in designated areas and lying flat.
– The floor is free of equipment not in use.
– Floors are cleaned free of excess dust.
– No expired IPA bottles.
– All Roller doors are closed when not in use.
– Materials to be used for Shrouding are stored in an orderly manner.
– Pallets of Goods to be Shrouded correctly stored and labelled.
v. Flammable goods store
– The flammable store is locked when not in use. Keys are not left in the door.
– The area is used for storage of flammable goods only.
– No storage of goods on the floor.
– No waste in the area.
– IPA bottles are stored safely with correct labeling and within the expiry date.
vi. Bulk store
– All components/products/packing materials are appropriately labeled and stored in allocated areas.
– All racking and storage areas are labeled clearly.
– All rejected stock is placed into the correct storage location.
– All batch numbers are separated and clearly labeled.
– All roller doors are closed when they are not in use.
vii. Waste area
– The ground area is free of waste.
– All waste is in the correct waste disposal area.
– Equipment is stored appropriately and does not block access ways.
– Waste from the laboratory is adequately labeled and segregated.
Good housekeeping, which essentially means “Everything in its place,” minimizes the opportunity for goods to be mixed up, reduces the possibility of dispatching or issuing incorrect goods, and improves warehouse safety.
In addition to good housekeeping, warehouse personnel should pay attention to safety rules, including what type of safety clothing should be worn.
Conclusion
The pharmaceutical warehouse is not just a storage facility; it plays a critical role in maintaining product quality and safety.
In this article, we have highlighted important GMP rules for keeping pharmaceutical warehouses in perfect condition and safely storing and distributing raw materials and finished products.
This, in turn, ensures that your manufactured medicines reach patients in optimal condition.
From receiving all important raw materials to distributing finished products, pharmaceutical warehouses adhere to stringent Good Manufacturing Practice (GMP) standards. GMP rules help to maintain strict control over every aspect of the process.
Key considerations, such as product status control to prevent mislabeling errors, designated storage locations to maintain product integrity, complex material handling, temperature & humidity controls, and stringent cleanliness protocols to prevent contamination, underscore the critical nature of the warehouse’s operations.
Pharmaceutical warehouses face multifaceted challenges. They must contend with temperature-sensitive biopharmaceuticals, navigate complex inventory management systems, and mitigate risks associated with poor storage conditions and rough handling during transportation.
However, these challenges can be effectively addressed through expert management, comprehensive personnel training, and unwavering compliance with regulatory guidelines.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
Customer Service Process for Quotations and Order Processing
How to effectively control packaging materials in pharmaceutical
How to perform periodic review of systems and processes in pharmaceuticals?







Thank you for your response! I’m grateful for your willingness to engage in discussions. If there’s anything specific you’d like to explore or if you have any questions, please feel free to share them. Whether it’s about emerging trends in technology, recent breakthroughs in science, intriguing literary analyses, or any other topic, I’m here to assist you. Just let me know how I can be of help, and I’ll do my best to provide valuable insights and information!
useful information helping to learn more about a career in logistics.
OK.. Good for use.