Guidance Summary 001 - 010
Guidance 001 Summary - Analytical Test Method Validation - General Guidance
This guideline provides guidance for the validation of analytical test methods. These analytical test methods include those tests which evaluate API Raw Materials, In Process samples (e.g. reaction monitoring) and early intermediate materials (prior to the introduction of the first critical intermediate).
- Limit tests (impurity, solvent OVI, clarity or extraneous matter tests).
- Quantitative tests for impurity content.
- Reaction Completion.
- Potency (assay) method for raw materials or intermediates.
- Tests for physical properties.
- Identification tests.
For new test methods, the type of test, speed of turnover of results, simplicity and criticality to the method is developed and the validation exercise is begun.
Once execution of the method validation has begun, deviations and experimental failures should be documented in the method validation report.
It is recommended that sites have local site procedures for performing test method validation. For directly linked to the method’s intended use.
Method Validation Summary Report:
It is recommended to analyse the experimental results and prepare a Method Validation Summary of the findings.
These method validation summaries may include but are not limited to:
For higher risk methods, at least two reviewer signatures are recommended to be obtained for the Method Validation Summary to be approved.
Test Raw Data or Reference to Raw Data.
Guidance 002 Summary - Analytical Test Method Validation Risk Assessment and Prioritization
This procedure provides guidance for the validation of analytical test methods.
- Registered analytical test methods.
- Methods used to test a critical attribute.
- Methods used to monitor a critical process parameter.
Methods performed during a processing step that are redundant with a validated method used to test the isolated intermediate or material.
Risk Assessment and Prioritization:
To ascertain which raw material, in-process control (IPC) and intermediate test methods require validation, it is suggested that a documented Risk Assessment be carried out on test methods currently utilized at an API site. This risk assessment approach can be used to evaluate legacy or new test methods.
Methods may generally be grouped from low risk to high risk.
Evaluation Process:
Conduct a cross functional and documented analytical test method evaluation based upon an understanding of the test data utilization by the Site Quality Team and other site functions.
Guidance 003 Summary - Analytical Test Method Validation - System Suitability
This guidance provides guidance for the validation of analytical test methods. The tests which evaluate API Raw Material, In-Process Control and Early Intermediate Material Tests.
The method validation exercise may include statistical interpretation of data to provide adequate justification for reduced System Suitability Testing and numbers of standard and sample injections. Suggested System Suitability recommendations are established in Appendix 1 for different types of API “In-Process testing”.
Recommended SST Data:
System suitability criteria from compendial general chapter methods may be used for some test methods but should be evaluated against the intended use of the test method as to applicability.
Minimum criteria should be that there is baseline resolution between the peaks.
Peak symmetry, as measured by the tailing factor, may be of importance to report, especially in impurity testing. If tailing is too extensive, it may mask other impurities. Other pharmacopoeias should be consulted if required, however, the US Pharmacopoeia recommends that to determine system suitability % RSD, 5 replicate injections if the % RSD is 2.0 or less and if the % RSD is greater than 2.0, six replicate injections are recommended.
Recommended SST Criteria:
The acceptance criteria used should assure adequate precision and specificity for the intended use. One approach is to set chromatographic SST criteria based on data collected during a validation exercise. Equivalency may be demonstrated as follows.
Guidance 004 Summary - Analytical Test Method Validation - Precision and Accuracy
This procedure provides guidance for the validation of analytical test methods. Guidance for precision and accuracy testing is provided, including guidance on reproducibility, repeatability, intermediate precision, and acceptance criteria for accuracy and precision.
Precision
Reproducibility:
Reproducibility is not normally performed during method validation. Reproducibility expresses the precision between laboratories and is assessed by means of an inter-laboratory trial.
Repeatability:
Repeatability expresses the precision under the same operating conditions over a short interval of time. Repeatability is also termed intra-assay or within run precision.
Accuracy
Accuracy may be established across the specified range of the analytical procedure.
Recommended Accuracy Data:
Percent recovery is calculated for each reportable value as defined in the method. The average percent recovery may be calculated at each level and compared to the acceptance criteria.
Recommended Accuracy Criteria:
expected specification range are important parameters. See Tables below for recommended acceptance criteria.
Statistical Basis for Acceptance Criteria for both Accuracy and Precision:
For impurity methods, the accuracy of the impurity determination can be either determined concurrently with the method precision recovery of spiked impurities or by spiking the impurity into a sample at approximately the Quantitation Limit (QL), 100% and 120% of the specification limit. Table 1: Recommended Criteria for Precision and Accuracy – Higher Risk Test Method Impurity
Impurity Spike Precision Accuracy
The table below summarizes the recommended target criteria for accuracy and precision for major component assays. The recommended precision criteria for Assay methods depends upon the intended use of the method. Higher risk methods are recommended to utilize more conservative acceptance criteria (+/-3 standard deviations) as outlined in Table 2. Medium risk methods are recommended to utilize +/- 2.5 standard deviations and Lower risk methods to use +/-2 standard deviations.
The criteria are categorized by actual or anticipated specification limits of the product.
Methods that meet these criteria are considered sufficiently accurate and precise to support the product specification limits. More exact criteria for accuracy and precision, based upon process capability indices, can also be used.
These criteria define a method as suitable if its mean recovery +/-3 standard deviations (typically intermediate precision) for higher risk methods fall within the specification limits.
For accuracy, the target criteria apply to the overall mean recovery at each level tested. The target criteria for precision for a higher risk method would be RSD<1.7% for both repeatability and intermediate precision. The accuracy criteria would be overall mean recovery within 97.5-102.5%.
The precision criteria are based upon the use of standard deviation prediction limits derived from the overall average and intermediate RSD. The prediction interval for higher risk methods (using 3 standard deviation) defines a range that captures nearly all (>99.7%) future results from the method. The prediction limits are compared to the product specification range. If the prediction limits fall within the specification range then the method is sufficiently accurate and precise to support its intended use (releasing product against specifications). The choice of the 3 standard deviation range for precision for higher risk methods is driven by two factors. Methods that meet the prediction limit criteria have less than a 0.3% risk of failing to meet specifications due to method noise alone. For higher risk methods the precision criteria represent the maximum RSD (rounded to 1 decimal place) that still allow 3 standard deviation prediction limits to fall within specification limits if the assay is unbiased – essentially the criteria are equal to 1/6 of the specification range. The accuracy criteria allow the method bias to be half as large as the specification range. For medium risk methods a 2.5 standard deviation precision criteria was chosen, and for lower risk methods a 2 standard deviation precision criteria was chosen.
Guidance 005 Summary - Analytical Test Method Validation - Quantitation and Detection Limit
Guidance for quantitation and detection limit testing is provided, including approaches, recommended data and acceptance criteria.
Quantitation Limit
The procedure is a non-instrumental or instrumental method. Determination of quantitation limit is not normally required for assay or identification tests.
The quantitation limit is generally determined by the analysis of samples with known concentrations of analyte and by establishing the minimum level at which the analyte
Based on Signal-to-Noise Approach:
Samples with known low concentrations of analyte with those of blank samples and by signal-to-noise ratio is 10:1.
Quantitation limit may be constructed in the validation documentation based on the calibration
The actual limit of quantitation would still be presented in numerical terms relevant to the assay method based on the discussion.
The quantitation limit (QL) may be expressed as: QL = 10 σ/ S where, σ= the deviation of the response; S = the slope of the calibration curve.
The residual standard deviation of a regression line or the standard deviation of y-intercepts of regression lines may be used as the standard deviation.
In all cases, the quantitation limit can be subsequently validated by the analysis of a suitable number of samples known to be near or prepared at the quantitation limit or reporting level.
The quantitation limit and the method used for determining the quantitation limit should be presented. For validation of the actual quantitation limit or reporting level:
Detection Limit
The detection limit is estimated as the concentration that corresponds to 3 times the standard deviation of the responses for the six injections.
A signal-to-noise ratio between 3 or 2:1 is generally considered acceptable for estimating the detection limit.
The detection limit (DL) may be expressed as: DL = 3.3 σ/ S where, σ= the standard Determination of detection limit is not normally required for assay or identification tests.
It is recommended that the detection limit and the method used for determining the detection limit should be presented. It is suggested that for limit tests, the detection limit should be less than one-half the specification limit where technically feasible. The detection limit should be less than the required reporting level.
Guidance 006 Summary - Analytical Test Method Validation - Linearity, Range and Specificity
This procedure provides guidance for the validation of analytical test methods. These analytical test methods include those tests which evaluate API Raw Materials, In Process samples (e.g. reaction monitoring) and early intermediate materials (prior to the introduction of the first critical intermediate).
During method development, selection of the assay range is linked to its linearity.
Recommended Linearity Data:
Concentration range of interest be tested. Test the standard solution using the method conditions.
Rationale to establish linearity using less than 5 different concentrations may be provided by considering the specification range (i.e. if the specification range is sufficiently narrow).
Recommended Linearity Acceptance Criteria:
When inferring accuracy from a linearity study, linearity could be considered acceptable if results, as compared to a standard, meet the accuracy criteria. Suggested acceptance criteria (for API Raw Material, In Process Control, and early intermediate material tests) for an acceptable linear relationship may be a test method having a minimum correlation coefficient (r) of > 0.95.
Range:
The range is the interval between the upper and lower levels of analyte concentration for which acceptable linearity, accuracy (recovery), and precision are obtained. The range should include at least five points to establish linearity.
For the assay, the ICH range is normally from 80% to 120% of the test concentration.
For determination of an impurity; the range of concentrations used to evaluate the linearity should consist of the quantitation limit and at least 120% greater than the concentration that would be the impurity specification limit.
More solutions may be evaluated if the linearity range must be extended.
Guidance 007 Summary - Analytical Test Method Validation - Robustness
This procedure provides guidance for the validation of analytical test methods. These analytical test methods include those tests which evaluate API Raw Materials, In Process samples (e.g. reaction monitoring) and early intermediate materials (prior to the introduction of the first critical intermediate).
Critical parameters effecting response factors, if used in the method, should be identified and characterized during robustness testing.
Parameters that can be used for test method robustness:
Solution Stability Experiments:
It is recommended that sites perform solution stability experiments.
Approach 1:
As directed by the test method, prepare standard and sample aliquots and analyze them. The test samples are allowed to stand, under normal conditions of test (e.g., at room temperature), for a minimum length of time equivalent to the maximum expected use time, (typically 24 hours to one week). Sample and/or standard stability are demonstrated for more than 24 hours if applicable. If possible, analyte stability is demonstrated over a time period that slightly exceeds the stability time period indicated in the test method.
Approach 2:
For standard stability for a low level impurity method, two different stock preparations of equal concentration are prepared (a1 and b1) and diluted separately to the same solution concentration (a2 and
b2). Six (6) injections of standard check solution “a2” and three (3) injections of standard check solution “b2” are performed. From each set of injections calculate the mean peak area response for the analyte main peak then calculate the standard check using the following equation.
Check = Mean Area STD “a2” x Concentration STD “b2”(µg/ml) x 100 Mean Area Std “b2” x Concentration Std “a2”(µg/ml)
Approach 3:
For a chiral HPLC method, solution stability is assessed using an injection and analysis of the sample of the appropriate test material at the following times after preparation.
Approach 4:
For TLC where the sample is required to be analyzed immediately, the standard only is analyzed and the intensity should be the same as at t=0 and the plate should not have new spots.
Approach 5:
For an HPLC method where the standard peak is a retention time marker only, the criteria for solution stability is for the main peak to be present and no new ones eluted. Repeatability studies cover the range of typical sample preparation time therefore sample solution stability is combined with repeatability studies.
Guidance 008 Summary - Calculations of Residue Limits for Drug Product for Equipment Cleaning
This guideline provides equations and examples for calculating the Maximum Allowable Residue (MAR), and Residue Acceptability Limits (RAL) for Drug Products and Non-Therapeutics.
Examples are provided for determining the acceptable equipment cleaning residue limits for therapeutic drug products (MART and RALT) and for non-therapeutic ingredients (MARN and RALN). For the therapeutic drug products both single product combination (Product A to B) and multiple products combination examples are given.
The attached Appendices give equations and example calculations for therapeutic drug product cleaning limits for a solid oral dosage form (tablet), creams/ointment and ophthalmic product. In Case-1 therapeutic example it is included the calculation of Maximum Allowable Residue (MAR) limits using two formulas; dose MART and toxicity MAR. It also includes an example for determining the worst case limit for shared equipment using multiple products. Example for residue limits calculation of CIP® 100 detergent, a non-therapeutic ingredient is also given.
Guidance 010 Summary - Product and Equipment Grouping and Worst Case Product Selection
Equivalent Cleaning Procedures
Any plan to group products or equipment for the purpose of cleaning validation should be based on the premise that the items grouped share the same cleaning procedure. the results of the cleaning validation across all those procedures.
Cleaning agent and cleaning agent concentration
Product Grouping
For the purpose of cleaning validation a group of related products to be identified and a single product selected as ‘worst-case’ or representative of the product family The rationale for the grouping must be documented. Types and examples of product grouping include:
Types and examples of product grouping
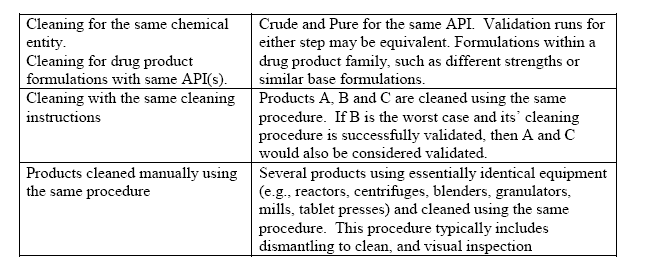
Once products are appropriately grouped, the worst-case product or products can be selected from among the group for the purpose of executing the cleaning validation protocol. ‘worst-case’ challenge to the cleaning procedure. Challenge products for the cleaning procedure used for products A, B, C, D and E.
The same cleaning procedure is used for two or more groups of products. Each worst-case product within each group should be subjected to the 3 validation cleanup requirement, unless a rationale is documented and approved by the Quality Authority that the worst-case product of one particular group is clearly a worst-case product for all groups.
Selection of the most difficult to clean product/process should be documented.
