
Equipment cleaning procedure in pharmaceutical, Do’s and Don’ts
- Published on: Jul 14, 2023
“It looks clean. Therefore it must be clean.”
This is not necessarily true for equipment cleaning in the pharmaceutical industry.
Equipment used for processing medicines is one of the top sources of contamination if not cleaned regularly. Some kinds of contamination are often difficult to detect by physical inspection. Also, laboratory testing for potential contaminants after every use of a piece of equipment is generally not practical, economical, or reliable.
You will need properly validated equipment cleaning procedures and a well-defined cleaning and verification regime to ensure your products are free from unnecessary contaminants.
In this article, we will discuss important aspects of equipment cleaning that are required during pharmaceutical manufacturing. Equipment cleaning validation protocol was not discussed intensely in this article, which was covered elsewhere in this blog.
Most importantly, we will demonstrate important elements of cleaning, do’s and don’ts, that will guide you in developing equipment cleaning procedures.
Once you apply the basic rules in developing your cleaning procedure, you will move one step ahead in assuring product quality and safety.
Importance of equipment cleaning procedure in pharmaceutical
Equipment cleaning is one of the fundamental activities of good manufacturing practice. Mainly cleaning the equipment that comes in contact with other products during manufacturing.
It is almost certain that a piece of unclean equipment will contaminate the next batch to be processed somehow.
In the pharmaceutical business, you must be aware of GMP rules emphasizing the effective cleaning of equipment that comes into direct or indirect product contact.
The GMP rules also require you to develop written equipment cleaning procedures to justify that equipment is clean during storage and before use.
Pharmaceutical equipment should be stored in a clean and dry place before being used in the next batch of products.
If you do not give particular attention to storage conditions, it is possible that the cleaned equipment can be re-contaminated from environmental exposure.
For example, contamination due to pests, dust, metal, soil, or foreign material if not fully covered.
Another way that equipment can be contaminated in storage is by leaving equipment wet, which can cause the growth of microbial organisms.
You have to develop an effective cleaning procedure that removes chemical and biological contaminants.
Regulatory requirements on equipment cleaning procedure
US FDA CFR 211, Subpart D—Equipment, Sec. 211.67 sets out the requirements you must implement during equipment cleaning and maintenance. Following are some of the rules you must consider.
(a) Equipment and utensils shall be cleaned, maintained, and sanitized at appropriate intervals to prevent malfunctions or contamination that would alter the drug product’s safety, identity, strength, quality, or purity beyond the official or other established requirements.
(b) Written procedures shall be established and followed for cleaning and maintaining equipment, including utensils used in manufacturing, processing, packing, or holding a drug product.
These procedures shall include, but are not necessarily limited to, the following:
(1) Assignment of responsibility for cleaning and maintaining equipment.
(2) Maintenance and cleaning schedules, including, where appropriate, sanitizing schedules.
(3) A detailed description of the methods, equipment, and materials used in cleaning and maintenance operations and the methods of disassembling and reassembling equipment as necessary to ensure proper cleaning and maintenance.
(4) Removal or obliteration of previous batch identification.
(5) Protection of clean equipment from contamination before use.
(6) Inspection of equipment for cleanliness immediately before use.
(c) Records of maintenance, cleaning, sanitizing, and inspection shall be kept as specified in 211.180 and 211.182.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Difference between equipment cleaning and sanitation
Cleaning is the removal of visible and microscopic contamination by dirt, extraneous matter, or product residues by mechanical or physical means. Cleaning is usually followed first by visual inspection and then by laboratory testing to verify that the cleaning continues to be effective.
Effective cleaning usually requires using cleaning agents such as detergents and solvents, which are used under specified pH, temperature, time, and solvent concentration conditions.
A cleaning concern is whether or not the cleaning agent will harm the equipment’s life or the product’s quality.
Sanitation, on the other hand, is the reduction of microbiological contamination. It is usually achieved by using chemicals. Heat steaming and vigorous mechanical action, such as scrubbing, can also achieve it.
Cleaning may result in a partial reduction of the microbial load. However, not all microbial loads can be destroyed by cleaning themselves.
If sanitizing chemicals are used, it may be necessary to remove the sanitizers’ residues by further cleaning. It is, therefore, very important to use the specified amount of sanitizing agent.
Several factors determine how effective sanitizing will be. These include:
– The number and types of microbes.
– The type of material containing the microbes.
– The concentration, temperature, and pH of the sanitizing agent.
– The length of time you sanitize.
When should pharmaceutical equipment be cleaned?
The main objective of cleaning pharmaceutical equipment is to prevent cross-contamination when manufacturing products such as steroids, hormones, antibiotics, cytotoxic products, and potentially pathological biological materials.
These materials, even in minute amounts, may have a significant effect on the body, and it is important to isolate them from the environment during manufacture and remove their residues from equipment during cleaning.
If you manufacture any of these products, ensure that you are fully aware of the handling and equipment cleaning rules.
Why is surface cleaning of pharmaceutical equipment important?
Surface cleaning of pharmaceutical equipment is important to remove or reduce contaminants to an acceptable level.
During production, the surface of pharmaceutical equipment comes in prolonged contact with matters from one product that are considered contaminants for the next batch if the product is not the same or the same product with lower strength.
These contaminations can be categorized as:
1. Chemical contamination:
Chemical contamination occurs when the product becomes contaminated with other materials or products. This can potentially occur when different products are processed in the same equipment or when materials such as cleaning agents and lubricants come into contact with products or product surfaces.
The way to avoid chemical contamination is by following written cleaning procedures exactly. Often, the steps and conditions in the procedures have been validated to ensure they are effective.
Changing the steps or conditions may make the cleaning ineffective.
2. Environmental contamination:
Different manufacturing areas within the facility carry different levels of contamination risk to the product.
There is a higher risk of contamination in areas where the product is exposed to the environment, for example, in the dispensary, formulation areas, and bottle-filling rooms.
Air control (usually filtered) is required, and frequent facility cleaning and environmental monitoring are recommended.
In other areas, such as secondary packaging or material storage areas, the product isn’t exposed to the environment, so there is a lower risk of contamination.
The less environmental air control is necessary, the more frequent cleaning is reduced, and environmental monitoring is minimal.
3. Biological contamination:
“Biological contamination” refers to contamination by bacteria, yeasts, molds, viruses, or any other microorganisms that may be present in the product. This also can include residues from dead micro-organisms or endotoxins, which can contaminate injectable products.
Biological contamination’s origins vary but include the environment, personnel, raw materials, contaminated water, and poorly cleaned or wet equipment.
While cleaning reduces biological contamination, sanitizing agents play an important role in killing microorganisms.
What are the common contaminants found in pharmaceutical production?
Production areas inside pharmaceutical plants inherently generate many types of residues, which are also contaminants if not intended to be used in a product.
Pharmaceutical equipment is one of the primary sources of introducing these contaminants to new batches of products.
Some potential contaminants or residues can include:
– Cleaning agents
– Denatured product
– Denatured excipients
– Heat-denatured residues (with biological products)
– Endotoxins or pyrogens
You should avoid cleaning equipment or materials that shed particles, raise dust, produce aerosols, or otherwise generate contamination where possible. These include compressed air, bristle brushes, fiber-shedding cloths, and certain designs of floor-scrubbing machines.
Vacuum or wet cleaning methods are preferred. Vacuum cleaners or polishers should be fitted with fine dust filters.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Common equipment vs dedicated equipment
A common question regarding equipment cleaning is,
“Does the equipment have to be cleaned if it’s going to be used again for the same product?“
The short answer is yes.
However, the depth of cleaning may be different depending on whether the following batch is the same strength of the same product, a different strength of the same product, or a different product altogether.
Each of these, in turn, will require more intensive cleaning.
Common equipment is equipment used for more than one type of product. You should completely clean and sanitize common equipment before every use, if necessary, in order to prevent the carryover from the earlier product to the next product.
Dedicated equipment is equipment used to manufacture only one type of product or for a run of batches (a campaign) of the same formulation.
Cleaning requirements for dedicated equipment may be less than those for common equipment, provided the company has documented either the maximum number of batches or the maximum time that can elapse before complete cleaning must be done.
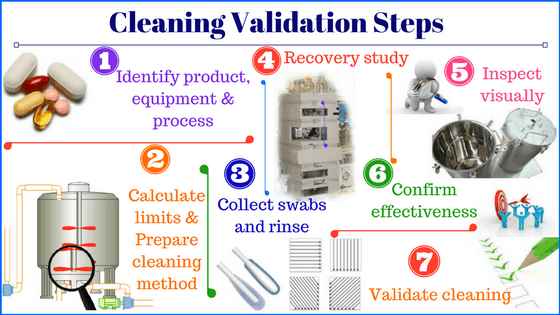
Four fundamental elements of equipment cleaning procedure
The GMP rules are very explicit regarding the requirement to clean and sanitize equipment and the manufacturing facility.
Since most residues cannot be seen nor reliably tested for, the company’s standard procedures and cleaning records are relied upon as evidence of cleaning.
1. Standardize equipment cleaning procedures
GMP requires the cleaning procedures to be fully documented in written procedures. These procedures are initially validated (shown to be effective) specifically for an item of equipment or an area.
Once validated, the procedures are then published and used.
2. Cleaning validation
You must validate all cleaning procedures (shown to be effective) under specific conditions of use. These conditions of use are specified in the written equipment cleaning procedure.
If employees alter the conditions of use, the cleaning methods may be “invalidated” and may not be effective.
For example, it would seem logical that adding more sanitizer than the procedure requires would make cleaning faster or better. This is not always the case. Adding additional sanitizer may alter the pH of the cleaning agent and make it less effective.
The relevant GMP rules are to only clean under validated conditions, and to follow procedures exactly.
3. Cleaning conditions
The written procedures describe the required conditions under which cleaning is optimal. These conditions may include a range of the following:
– Strength of the cleaning or sanitizing agent (e.g., 2.0% v/v)
– The type of water or solvent to be used
– The pH of the cleaning agent
– The temperature of the water to be used
– How much to dismantle equipment before cleaning commences
– The contact or residence time for the sanitizing agent on the surface
– What agents to use to clean off the sanitizing agent
– What standard of water to use in the final rinse (GMP, for example, requires purified water for injection as the final rinse)?
4. Cleaning records
One essential GMP rule is the keeping of detailed cleaning records.
Cleaning records provide proof that cleaning took place and evidence of the cleaning outcomes.
For example, whether the surfaces are visually clean or the results of rinse water tests. The records must also identify who did the cleaning and when and must be signed.
For automatic cleaning procedures such as clean in place (CIP), the cycle conditions are usually automatically monitored and the conditions recorded.
Cycle conditions may include the temperature, flow rate, time, concentration of solvent, and solvent agitation time. Often, CIP systems have alarms when something goes wrong.
The completed and signed records should be attached to the records since they provide the only objective evidence that cleaning has occurred.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
How to design effective equipment cleaning procedure?
It is important that you develop cleaning procedures for your equipment that are used for manufacturing medicinal products in order to ensure there is no contamination or carry-over of ingredients between two batches.
You must clean equipment using written Standard Operating Procedures (SOP) and instructions with an attached cleaning checklist. The production and quality assurance teams must approve these documents.
When developing a cleaning procedure, you have to focus on a few important cleaning elements.
In the following section, we will discuss some of those elements. To make your cleaning procedure effective, try to combine some or all these elements as necessary.
Element 1: When does your equipment come into product contact?
Pharmaceutical equipment, both major and minor, that comes in contact with the product during manufacturing, sampling, handling of in-process material, or starting material must be cleaned.
This should include, and not be limited to:
– Changeover cleaning.
– Interval cleaning during a campaign, as necessary, or
– Dedicated equipment cleaning at the end of a campaign.
– Equipment disassembly may be required to clean or to verify cleanliness.
Use and cleaning History must be determined for product contact equipment used by third parties or another facility (e.g., trials, rentals, borrowed).
The history must be documented and approved by the quality assurance. The removal of previous product residues must be verified before any use.
You’ll need to maintain product contact history in such a way as to show that the equipment was not used for and does not contain potential contamination from objectionable materials (e.g., penicillins or other beta-lactams, pesticides).
Element 2: How to validate the equipment cleaning procedure?
Validation of equipment cleaning procedure in pharmaceutical proves that the cleaning procedure will consistently remove residues from previous products and cleaning agents and reduce the microbial population to a safe and acceptable level.
The focus on cleaning validation commenced in the early 1990s when the FDA published the Inspection Guide to Equipment Cleaning and Cleaning Validation.
The increased manufacture of potent and new biotechnology products with high potency in low concentrations prompted this guidance. It was clear by then that any of these products left on equipment could cause major contamination risks.
Equipment cleaning validation becomes very important when companies handle potent or toxic drugs.
Validation of the cleaning procedure ensures the efficacy of cleaning in the following ways:
– Defines the products that can be made in the equipment.
– Determines the maximum time allowed before the tank/equipment must be cleaned.
– Defines the cleaning agent to use and its correct volume and concentration.
– Verifies the temperature of the cleaning process.
– Verifies the length of time needed for cleaning.
Cleaning and sanitizing validation verifies that the specific cleaning procedure will be effective for a specific range of products in specific equipment under the specific conditions described in the procedure.
Any changes to these parameters may make the cleaning/sanitizing ineffective.
You should establish the Maximum Allowable Residue (MAR) and Residue Acceptability Limit (RAL) for equipment cleaning based on the empirical data and the approved calculation method before use.
Validate the swab or rinsate sampling methods through the use of recovery studies. The analytical methods used to test for residue must also be validated.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Element 3: Your cleaning procedure must reduce cross-contamination.
You must design the equipment cleaning procedure to prevent Cross Contamination, including microbiological contamination. Where applicable, conduct risk assessments to reduce residues on all product contact surfaces to acceptable levels.
Contamination of a starting material or a product by another material or product must be avoided.
This risk of accidental cross-contamination arises from the uncontrolled release of dust, gases, vapors, sprays, or organisms from materials and products in process, from residues on equipment and operators’ clothing.
To prevent cross-contamination Equipment cleaning is critical to product quality. Cleaning is as much a technical task as the formulation of products. So, it requires the same attention to detail and compliance with GMP.
The same principles apply to equipment cleaning as they do to facility cleaning:
– Follow all written cleaning SOPs,
– Maintain records of cleaning.
– Use only those cleaning agents that have been approved by QA.
– Store cleaning equipment in dry conditions and clean the cleaning equipment regularly.
With equipment, a tagging system should identify the equipment cleaning status.
Equipment may have several areas where cleaning is difficult or where product residues tend to build up during processing.
Element 4: How to choose cleaning agents for equipment cleaning?
Cleaning agents and equipment must be carefully evaluated before being used in a manufacturing facility.
The choice of cleaning and sanitation agents is a decision made by quality, so it should be ensured that only quality-approved cleaning agents and equipment are used during the cleaning.
GMP rules state that cleaning procedures must indicate the nominated strengths of cleaning agents and that cleaning agents cannot be changed without approval.
The selection of a cleaning or sanitizing agent is dependent upon:
– The previous product type, for example, a cream or powder
– Compatibility with equipment to be cleaned
– Ease and safety of use
– Ease of removal of residues
– Required contact times on equipment
– The targeted microbial population
Element 5: What choice do you have on cleaning and sanitizing agents?
Different sanitizing agents will be effective only in certain circumstances.
1. Sanitizer
Proper use reduces bacteria by>99.9% if used on pre-cleaned surfaces. Pre-cleaning is always required to reduce bioload and dirt.
2. Disinfectant
Proper use results in 100% killing of vegetative bacteria, target viruses, and target fungi. Pre-cleaning may or may not be required, but it is considered a pre-requisite.
3. Sterilant
Proper use results in 100% kill of all micro-organisms, including bacterial spores. Pre-cleaning is always required to reduce bioload and dirt.
4. Sporicide
Proper use results in a 100% kill of all bacterial spores.
Following is a list of commonly used sanitizers you can choose to use. However, you must validate the condition and efficacy of their use by conducting trials.
Always remember to include only approved sanitizers in your cleaning procedure.
– 70% v/v alcohol (for surfaces)
– Quaternary ammonium compounds (QUATs)
– Phenolics
– Sporicides (for killing spores)
– Chlorines (such as hypochlorite)
– Hydrogen peroxide/peracetic acids
– Sodium hydroxide (cleaning & partial sanitizing)
Element 6: How to choose disinfectants for equipment sanitizing?
Disinfectants must be used in strict accordance with the cleaning procedure. They should never be topped up, used, or stored without labels and expiry dates.
Disinfectants are tested for their bactericidal activity against four standard cultures of micro-organisms, namely:
– Pseudomonas aeruginosa
– Vulgaris
– coli
– Staphylococcus aureus
As stated on the product label, the disinfectant is tested at the manufacturer’s recommended dilution.
The disinfectant dilution is passed or failed according to the extent of growth shown by the challenge bacteria. This is why making dilutions correctly during use is very important.
A common practice in the industry is to rotate between different sanitizing agents regularly (for example, monthly).
This intends to prevent the possible buildup of organisms with resistance to the sanitizing agent being used.
Element 7: How to choose good cleaning tools for equipment cleaning?
You can use a number of cleaning equipment in your cleaning procedure. Let’s find out which ones are good and which are not so useful.
i. Steel Wool: This is not a good item because steel wool sheds fibers and is very abrasive. It can cause fine surface scratches (even in stainless steel), providing an ideal home from micro-organisms.
ii. Nylon Scourer: Not a good item to use because scourers shed fibers and are very abrasive. They can create line surface scratches, which make cleaning more difficult.
iii. Wooden Brush: This is not a good item to use because wood is hard to dry, and the bristles are hard to clean and may break off. Brushes provide good habitats for microbes because they’re likely to be dirty and wet.
iv. Nylon Brush: This is a good choice because nylon brushes are easier to keep clean and dry and the bristles are soft and non-abrasive.
v. Non-fiber shedding wipes: This is a good choice because they will not shed fibers and are non-abrasive. Usually, they are used only once so they are very hygienic as well.
vi. Wooden mop: Not a good item to use because wood can splinter and is hard to dry. Wood provides a suitable habitat for microbes because it is likely to get dirty and wet. String mop heads also shed fibers so they are best avoided.
vii. Wet dirty rag: Not a good item to use because it is wet and dirty. No doubt it contains thousands of microorganisms.
viii. Steel-handled Squeegee mop: A clean squeegee on a squeeze mop with a plastic or metal handle is an effective cleaning item.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Element 8: Verification and visual inspection of cleaned equipment.
When you conduct routine cleaning verification, please make sure lighting is enough to facilitate visual inspection for residues. The equipment must be dry before inspection for areas to be visually inspected.
For changeover cleaning, When you conduct routine verification of cleaning of major equipment where 100% visual inspection is not possible, keep records of the following:
– Inspection to the extent practical to verify the equipment is visibly clean.
– Periodic monitoring (e.g., using swab or rinsate sampling method as per validation) at a frequency defined based on a documented and approved risk assessment (e.g., up to 2 to 3 years) of the probability of contamination and
– Approved justification of the rationale for the frequency of the periodic monitoring.
When you conduct routine verification of cleaning of major equipment where 100% visual inspection is possible, keep a record that the equipment is verified to be visibly clean (e.g., mills, filter housings, and tray driers).
For minor pharmaceutical equipment, routine verification of the cleaning process should include a visual inspection to verify the equipment is visibly clean.
Employ a qualified inspector other than the one who cleaned to verify that the equipment is visibly clean.
Element 9: How to verify the equipment is clean during a campaign?
When using commercial production equipment for a one-time product (e.g., clinical batches/lots), for which cleaning has not been previously validated, that equipment must be verified as clean by visual and residue testing.
Verification must be done after the first production and before the next use of the equipment.
Define and document the need for cleaning, type or level of cleaning, and cleaning frequency within the campaign.
Could you perform interval cleaning as required during a campaign?
Product changeover cleaning procedures must be validated for all product contact equipment used for multi-product production and sampling of drug products.
Perform a pre-use inspection immediately before using the equipment following a changeover cleaning or dedicated equipment campaign cleaning, and document (e.g., in the next batch or campaign record).
The pre-use inspection shall verify that the equipment:
– Has been properly assembled (e.g., via visual inspection or communication with the responsible personnel).
– Has been cleaned.
– Is visibly clean.
– All identification from the prior product has been removed.
– Cleaning records are verified for completion.
– Analytical results (if applicable) are satisfactory; and
– Maximum allowable time period between cleaning and next use was not exceeded (if applicable).
Element 10: How to confirm the status of cleaned equipment?
You must display the cleanliness status of each equipment unit or item using labels and signs or a centralized paper-based or validated electronic system.
The cleaning status can only be changed to cleaned once all monitoring results (when applicable) are satisfactory.
Examples of equipment cleaning status include, but are not limited to:
– Hold for Cleaning.
– Cleaning in progress, or
– Clean.
Always ensure equipment status should be apparent at all times, including during storage.
For example, this status tag on a vessel should indicate:
– Whether the equipment is clean or requires cleaning
– When the equipment was cleaned or is to be cleaned
– The previous product in the vessel
– When the equipment is due to be re-cleaned
Element 11: What records need to be kept as proof of cleaning?
For each of the major equipment, you must maintain logs of equipment cleaning, history of use, service, and maintenance. These should have proof of chronological entries on sequentially numbered pages or equivalent validated electronic systems.
Information to be recorded should include, and not be limited to, the following:
– Documentation of cleaning, use, and maintenance.
– Date of the activity.
– Start and finish times of the activity.
– Equipment use, including product name and batch numbers or lot numbers of each lot or batch processed in the equipment.
– Signature or initials of the individual performing the work; and
– Signature or initials of a second person verifying the work, where no other primary Records are in place to document the work.
Personnel responsible for equipment cleaning operations and sampling must be trained and qualified. Such training, along with evidence of training effectiveness, shall be documented.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Element 12: How to manage equipment hold time between cleanings?
You must take adequate measures to protect clean equipment from environmental or microbial contamination during storage.
After it is cleaned, the equipment should be stored with a protective cover to prevent contamination on storage.
Regardless of its cleaning status, equipment should always be examined before use to verify that it is clean and suitable.
Take the following steps during the equipment hold time:
– Store equipment dry,
– Close tank openings,
– Close tablet machine covers,
– Cap or cover open flanges, and
– Protect portable equipment (major and minor) from environmental exposure by sealing it in a protective bag or shrouding and storing it cleanly.
– Cleaned portable equipment must be stored, segregated from, in-use, and unclean equipment.
Following is a list of situations where you are required to decide whether to use or clean pharmaceutical equipment based on the risk of contamination.
1. Post-use / pre-clean:
Once you have completed batch processing, there must be a maximum hold time between post-use and the commencement of cleaning.
This is because, over time, any residues will start to “bake” onto equipment surfaces and will be much harder to clean off. In addition, bioburden may start to grow.
You must establish the maximum allowable hold time through a validation process. You must clean the equipment before the maximum hold time will elapse.
2. During the cleaning cycle (contact time):
During the cleaning cycle, your cleaning procedure should specify a minimum hold time for contact of the cleaning agents and sanitizers with equipment surfaces.
For cleaning and sanitizing agents to be effective, they must be in contact with the dirt and surface for a minimum period of time. You must validate this contact time.
3. Storage between use
Equipment must be stored dry and protected. GMP rules require that stored equipment must have an “expiry date” or maximum storage time before it needs to undergo re-cleaning.
Most companies have a simple rule:
“All equipment is inspected thoroughly before use, and any equipment that exceeds its maximum storage hold time is re-cleaned before use.”
You must evaluate the maximum allowable time intervals between use and initiation of cleaning. If this time period potentially impacts the ability to clean the equipment, then the time period must be specified and validated.
If the maximum allowable time intervals are exceeded, the equipment must be re-cleaned and inspected before use. If applicable, the equipment shall also be sampled (e.g., for potential microbiological contamination).
Document the re-cleaning and cleaning verification in the logbook, cleaning instructions, or checklist.
Element 13: Cleaning of water residue in hoses
A common problem in production areas is the cleanliness of transfer hoses.
Transfer hoses can be a major source of microbial contamination if left wet in storage. If a wet hose is reused later, it may transfer bio burden back into the tank on the first flush, causing microbial contamination of the next batch.
The specific GMP rules for hoses are:
– After cleaning, ensure that all hoses are detached from the equipment and fully drained.
– Where possible, store hoses vertically away from wash bays.
– Keep hoses off the floor.
– Periodically sanitize the hoses to keep them fresh.
– Flush the hoses before use where possible.
– Where hoses are dedicated to a product, make sure that they are properly identified as such.
Element 14: Use of water in equipment sanitation.
Water is often used in the cleaning and sanitation of equipment. Therefore, you should know that:
– Water is a good breeding place for micro-organisms.
– Bacteria can double in 15 minutes in water.
– Many water bacteria are harmful and cause consumer sickness or product spoilage.
– To protect the next product from microbial contamination, equipment must be stored dry.
– The final rinse of equipment must be in either purified water or water for injection so that the surfaces are not re-contaminated after the complete cleaning cycle.
Element 15: Equipment cleaning after service and maintenance.
You should conduct an evaluation after the completion of any maintenance or instrument calibration activities that require the opening or disassembly of equipment. Document the level of cleaning required for product contact equipment, if any.
Before being put into service, new equipment should be cleaned, and the equipment shall be verified as visually clean at a minimum. The cleaning and verification of cleanliness must be documented and approved before use.
You must be able to document and investigate equipment cleaning failures during routine monitoring (i.e., visual and/or analytical result failures) according to established procedures. An evaluation of the impact on validation must be included.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Conclusion
In conclusion, equipment cleaning, especially equipment that comes in contact with medicinal products, is absolutely necessary to avoid any risk of contamination.
Many medicinal batches are recalled each year by regulatory authorities worldwide due to actual suspicion of contamination. One of the primary causes of contamination is uncleaned or inadequately cleaned equipment.
Please take a look at all the cleaning elements we have listed in the post and assemble those while constructing the equipment cleaning procedure.
A robust equipment cleaning procedure and its validation process are essential to prove your equipment is adequately cleaned, ensuring product safety and purity.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.















need to buy this sop