Validation Guideline for Facility and Utility
- Kazi
- Last modified: August 18, 2024
This validation guideline for facility and utility aims to provide a clear statement of the scope, validation approach, and testing required for validating the facilities and utilities that are directly or indirectly involved in the manufacturing processes.
This validation guideline will apply to all GMP critical facilities and utilities.
Responsibilities for validation
It is the responsibility of the validation committee to review and approve the master validation plan, thereby approving the general approach and methods to be employed in the validation of the manufacturing facilities and utilities used throughout the manufacturing facility.
Technical Services
– The Technical Services department is responsible for establishing and maintaining facility/utility validation programs and developing and approving all guidelines, plans, protocols, and reports.
– Technical Services is responsible for verifying that all facility/utility validation programs comply with this guideline and current GMP practices.
Engineering
– Engineering is responsible for ensuring that validated utilities support all major GMP manufacturing equipment as applicable and that facilities are qualified.
Quality Assurance
– Quality Assurance is responsible for ensuring that the GMP aspects of the facility/utility validation program comply with relevant procedures and that critical parameters and report conclusions are supported.
Rationale for validation of facility and utility
Installation Qualification and Operational Qualification exercises assure, through appropriate performance tests and related documentation and records, that facilities and utilities have been commissioned correctly and that they will be reliable and within prescribed or specified operating limits.
The validation approach will be based on a risk assessment following the SOP for Quality Risk Management and the impact assessment outlined in the SOP Validation Impact Assessment Procedure.
Qualification will be performed if the utility directly affects product quality as described in this section. Qualification may not be required if the utility does not directly affect product quality.
Quality Assurance must approve the justification for not qualifying the utility. Facility Qualification will be performed in GMP areas only.
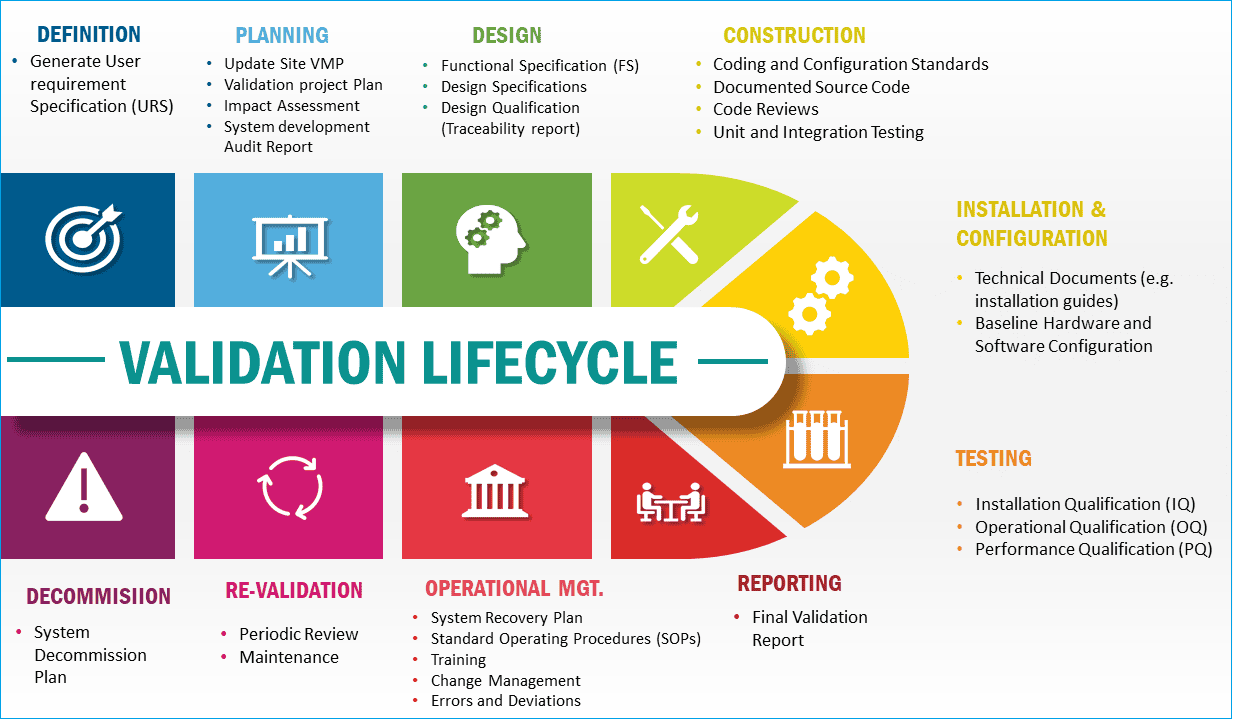
Validation Project Plan
A validation project plan (VPP) should be written for large-scale projects. VPPs represent dynamic, project-specific master validation plans, often accompanied by several appendices that change as the project progresses.
Design Qualification
The first element of the validation of the new facilities and utilities is Design Qualification (DQ).
The DQ documents that the design of facilities and utilities meets GMP, process user, and safety requirements and is suitable for its intended use.
The DQ process can be incorporated into the IQ or validation report.
Installation Qualification
Installation Qualification (IQ) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user requirements, and manufacturer recommendations.
Furthermore, IQ ensures that a record of the principal features of the facilities and utilities, as installed, is available and supported by sufficient documentation to enable satisfactory operation, maintenance, and change control to be implemented.
Installation Qualification (IQ) should be performed on new or modified facilities and utilities.
IQ should include an appropriate, but not limited to the following:
– Installation of piping, services, and instrumentation checked to current engineering drawings and specifications;
– Collection and collation of supplier operating and working instructions and maintenance requirements;
– Calibration requirements;
– Verification of materials of construction;
– Cleaning and passivation results;
– Pressure testing/weld checks/pipe slope isometrics;
– Safety requirements;
– Training.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Operational Qualification
Operational Qualification (OQ) provides documented evidence that the utility operates as intended throughout the specified design or anticipated operating ranges and complies with GMP, EHS legislation, and other relevant standards.
The utility’s critical operating parameters should be identified at the Operational Qualification stage.
The plans/protocols for the Operational Qualification should identify the studies undertaken on the critical variables, the sequence of those studies, the measuring equipment to be used, and the acceptance criteria to be met.
A simulated product may be used to conduct the Operational Qualification where applicable. Studies on the critical variables should include worst-case conditions.
All testing equipment should be identified in the protocol and calibrated before use.
All critical instruments will be calibrated before Operational Qualification.
Where sampling is performed, the specific sampling site must be identified in the protocol.
Commissioning performed following installation is not a substitute for OQ; however, if the commissioning is documented and witnessed/reviewed by the employee, the results may be used as part of the OQ.
Satisfactory installation and operational qualification exercises should permit a formal ‘release’ of the facility/utility for the next stage in the validation exercise.
This release should be documented after the validation exercise is completed.
Facility and Utility Validation Protocol
Protocols for each type of qualification will be written according to the site SOP.
Depending on the size of the system or item to be validated, IQ and OQ may be combined into one protocol and one report.
Review the manufacturer’s validation package where possible for new utilities and purchase if suitable. The validation team will make additions to the package as necessary to meet site quality standards.
As outlined in this document, the manufacturing site must pre-approve vendor protocols to meet the in-house requirement. A validation report will be issued for vendor protocols.
When there is more than one identical building or utility, each has to be qualified; however, identical protocols can be used.
Where operating manuals exist for a utility, protocols should reference these manuals.
Validation reports will be written for each protocol. Protocols written for specific utilities must adequately describe the intended use.
An environmental health and safety (EHS) risk assessment will be essential to any utility protocol.
The specific protocols for each item will describe and justify the acceptance criteria. Generally, the facility/utility must conform to GMP requirements, EHS standards, and applicable Australian standards.
Any deviations encountered during the validation study must be documented in the validation protocol/report and approved by Quality Operations.
For established facilities and utilities with critical aspects that need to be validated, gaps in qualification activities in the existing documentation shall be identified.
If there are gaps, remediation activities shall be developed and implemented.
Qualification of Utilities with Computer Systems
Utilities containing Programmable Logic Controllers (PLC) and/or computer systems must also conform to the SOP Computer Validation Guidelines.
Usually, the computer system is qualified as part of the utility qualification. Separate protocol(s) and report(s) are not required.
Maintaining the Validated State
Once validated, all facilities and utilities will be maintained in a validated state throughout their life cycle.
Modifications to or relocations of validated facilities and utilities must be authorized through the change control system. The modification or relocation will require full or partial requalification.
The change control system, re-qualification, training, SOPs, calibration, and engineering maintenance programs will achieve this.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Conclusion
In conclusion, this validation guideline for facilities and utilities within GMP environments outlines a comprehensive approach to ensuring that all critical facilities and utilities involved in manufacturing processes are validated and maintained in a state of compliance.
The guideline emphasizes the importance of a structured validation process, including Design Qualification (DQ), Installation Qualification (IQ), and Operational Qualification (OQ), to confirm that these systems meet GMP, safety, and operational requirements.
A multidisciplinary team, including Technical Services, Engineering, and Quality Assurance, supports the validation efforts. Each plays an important role in establishing, maintaining, and verifying the validation program.
Additionally, the guideline addresses the need for re-qualification following any modifications and stresses the importance of maintaining the validated state throughout the lifecycle of the facilities and utilities.
This ensures the continuous reliability and compliance of the manufacturing environment, ultimately safeguarding product quality and patient safety.
Please take a look at the following relevant articles for detailed examples of validation.
Concept of process validation in the pharmaceutical industry
A step-by-step guide to successful installation qualification (IQ)
How to perform operational qualification – step-by-step
Validation master plan (VMP) – when and how to create one?
What is computer system validation (CSV) in GMP
Process validation guidelines for formulated products

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.