Importance of acceptance criteria in analytical method transfer
- Kazi
- Last modified: March 13, 2025
Table of Contents
What is analytical method transfer?
Analytical method transfer involves relocating validated test methods from one laboratory to another without compromising the original validation attributes of the transferring method. Analytical method transfer can be a part of technology transfer between two GMP or GLP sites.
In the process of transferring a method, there are at least two entities. One is the transferring laboratory (TL) or sending laboratory where the method was initially validated and used.
Then there is the receiving laboratory (RL) where the new method needs to be validated to ensure the method will be reproducible and robust when applied for product testing later.
The transfer of validated analytical methods requires that the method performance does not change because of transfer activity.
That is, the same data quality can be generated in support of product quality at the receiving laboratory as generated in the transferring laboratory.
Analytical method transfer is necessary in almost all cases when a company acquires a medicinal product from another company under a quality agreement and both have a license to operate quality control laboratories for product testing.
Also, method transfer can occur between two contracted laboratories sometime within the same business or two unrelated businesses.
Although, the method transfer is not intended as a reconfirmation of the entire scope of the validation study. Rather, it is a supplement to the validation study that demonstrates the analytical method’s reproducibility, or inter-laboratory precision.
In this article, we will briefly discuss some essential elements of analytical method transfer, industry best practices, method transfer strategies, method transfer process workflow, and necessary considerations for a seamless transition.
In the later part of the article, we will explain how to determine testing patterns and acceptance criteria for analytical method transfer exercises sometimes abbreviated as AMTE.
We will discuss the best practice in identifying lots and number of samples required for testing and setting up appropriate acceptance criteria for laboratories during the method transfers.
Let’s dive in!
Understanding key elements of the analytical method transfer process
We already know that when it comes to method transfer, two main parties are involved. The transferring laboratory (TL) is the origin of the analytical procedure, while the receiving laboratory (RL) is the recipient.
According to USP <1224>, method transfer is defined as the documented process that qualifies a laboratory (RL) to use an analytical method originating from another laboratory (TL), regardless of whether it’s internal or external.
a. What are the responsibilities for transferring laboratory (TL)
Before initiating the method transfer, the TL must provide the RL with a comprehensive transfer package. This package includes:
– Evaluate the validation study to ensure compliance with analytical validation standards,
– Provide appropriate documentation pertaining to the method development, validation, post-validation studies, limits, equipment, analytical procedure, and purpose and timing for transfer to the receiving laboratory,
– Provide input into the transfer protocol and report,
– Provide training to receiving laboratory on the method,
– Participate in the transfer study,
– communicate and collaborate with the receiving laboratory.
– Analytical data,
– Previous method validation reports,
– Transfer reports,
– Relevant standard operating procedures (SOPs),
– Sample chromatograms,
– Quality control (QC) trends/stability data, and
– A list of any known problems and their resolutions.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
b. What are the responsibilities for receiving laboratory (RL)
The receiving laboratory’s responsibilities include but are not limited to:
– Evaluate the documentation provided by the TL to identify potential issues and evaluate resources and timing,
– Prepare and approve the analytical method transfer protocol and report,
– Complete GMP training for employees requiring working with the new method
– Perform transfer study,
– Initiate appropriate documentation required for routine use of the analytical method, and
– Communicate and collaborate with the sending laboratory.
c. What is the analytical method transfer protocol?
Properly transferring a method between two laboratories requires meticulous planning and a thorough assessment of various factors.
A method transfer protocol is created to formalize the transfer, outlining the objectives, scope, responsibilities, method specifications, acceptance criteria, documentation requirements, references, and approval process.
Typically, it is the QA management’s responsibility to ensure cGMP compliance during method transfer, review and approve the transfer protocol, report, and maintain communication between the laboratories.
d. Which method transfer strategies to choose from?
Your method transfer strategy should be tailored based on factors such as the type and validated status of the method, the intended product, and the RL’s experience in validating the method.
Evaluating these aspects helps you determine the appropriate transfer procedure. Considerations must also be given to the specific application of the method, such as release, stability, or in-process testing, etc.
By leveraging existing knowledge and experience, a method transfer can be simplified, especially if the RL has familiarity with similar methods or different product variations.
The following are three commonly known method transfer approaches used for the process.
1. Comparative studies:
In comparative testing, the same samples collected from the same lots are tested by the TL and RL and results are compared against the set acceptance criteria. Arguably this is the most used strategy during analytical method transfer.
Examples of the tests include assay, impurity, related substance, residual solvents (GC), particle size, etc.
2. Co-validation experiments:
This strategy is most useful when two or more laboratories are involved in method transfer. The RL takes part in validation studies especially intermediate precision study that is useful in assessing method reproducibility.
Examples of the tests include assay, impurity, and related substances.
3. Re-validation experiments:
In cases where TL is unavailable to take part in method transfer experiments, the only option available is for the RL to perform a re-validation of the test method.
In this option, the RL has to assess all the risks and mitigate those risks by repeating the test parameters and matching the results against the original validation data.
e. Ensuring method validation and suitability
The RL plays a crucial role in verifying the method transfer protocol and appointing competent personnel to execute the transfer process.
It’s essential to review the TL’s validation approach in accordance with current ICH Q2 guidelines and ensure that it covers the intended use of the method.
Checking if the results obtained by the TL meet the acceptance criteria is also vital.
Additionally, the RL must ensure the availability of necessary equipment, chemicals, and consumables required for the method transfer.
f. Setting up acceptance criteria for success
Defining acceptance criteria is a critical part of analytical method transfer.
Various approaches can be employed, such as adjusting acceptance criteria based on validation, process capability index (KPCI), or statistical equivalence tests.
Statistical equivalence tests are often applied for complex methods, while simpler methods may involve direct result comparisons.
The acceptance criteria should be well-defined to ensure a successful transfer.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
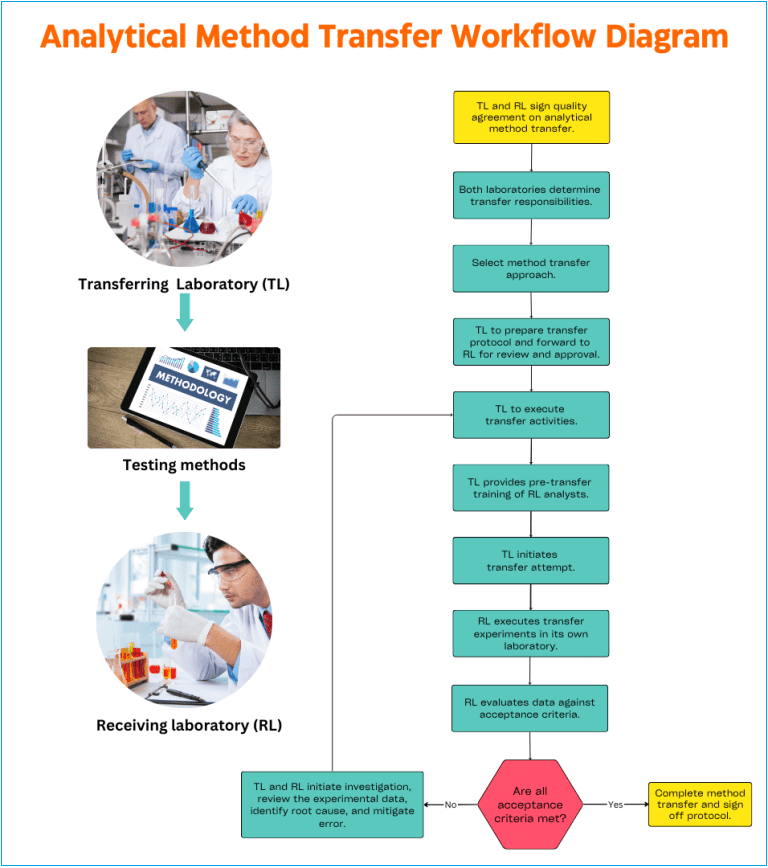
Analytical method transfer process workflow
It is not practicable to prepare one set of method transfer workflow that works in every transfer project when there are a lot of variables to consider. Each method transfer protocol should be devised carefully to meet the laboratories unique objectives, scope, and test method acceptance criteria.
Primarily, you should follow regulatory guidelines such as ICH Q2 and USP (1224) based protocols that should be correctly interpreted and followed.
However, there are some fundamental practices that you can follow during analytical method transfer exercises.
A. Company Quality Assurance management is responsible for ensuring cGMP compliance during the analytical method transfer. Quality Assurance must review and approve the quality agreement, the transfer protocol, the final transfer report, and certification. QA maintains communication between the laboratories.
B. In general, the transferring laboratory (TL) initiates the transfer activities. In the comparative or co-validation transfer approaches, the (TL) should comply with the responsibility to provide all pertinent information regarding the methods to be transferred to receiving laboratory (RL).
C. The provided information includes but is not limited to:
– Appropriate pre or post-validation studies,
– Analytical procedure,
– Detailed equipment list,
– Limits laboratory/reference standard information, and
– The purpose of and timing for transfer completion.
D. Upon delivery of the method-specific information, the RL reviews the information to identify potential issues, evaluate resource needs, determine the training needed, and prepare a transfer timeline.
E. The results of this evaluation are communicated to the TL. Issues already resolved should be documented in the transfer protocol.
F. A transfer protocol should be prepared using input from both the TL and RL. This protocol is to be approved by both the TL and RL as well as QA. The protocol should contain information such as:
i. An assessment of the appropriateness of a comparative study for the method,
ii. Details of the comparative study, if needed, including an appropriate sample size to demonstrate that a difference can be detected should one exist, acceptance criteria, details of the representative sample/s to be used and the manner of data collection, and
iii. Timing for completion of the transfer study.
G. If new equipment is needed in the RL, then installation and qualification of the equipment per corporate standards are to be completed prior to generating data for the comparative study.
H. TL should provide the necessary pre-transfer training of the RL. A familiarization period allows for the method to be performed as written to ensure the requirements of the procedure can be met.
I. Analytical transfer data generated in each laboratory must be collected as described in the transfer protocol.
J. Data generated in each laboratory should be statistically compared, if appropriate, per a transfer protocol and any internal guidelines to demonstrate reproducibility.
K. A method transfer is considered successful when the required acceptance criteria are met by both TL and RL.
L. After successful transfer, a transfer report must be prepared that includes the results of the transfer study, statistical analysis if appropriate, and a statement of the acceptability of the receiving laboratory to perform the analytical method.
M. If the acceptance criteria are not met, RL in cooperation with TL should initiate an investigation, review experimental data, identify the root cause of the deviation, and mitigate all errors.
N. Deviations from the transfer protocol must be justified. Notebook references to raw data must be included. This report is to be approved by both the sending and receiving laboratories as well as QA.
O. Written records shall be maintained such that the transfer of individual procedures and the historical sequence of subsequent changes can be reconstructed. These historical records shall be retained according to approved record retention schedules.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How to determine testing patterns and acceptance criteria for analytical method transfers?
The extent of testing during analytical method transfer should be commensurate with the method’s capability and its intended use.
That includes the review of specification levels, validation data, and historical performance for each method (where available) and determining the appropriate transfer criteria/limits.
Based on the intended use of the method, appropriate acceptance criteria can be determined which should include:
– Evaluation of inter-laboratory differences,
– System suitability,
– Precision,
– Limit of Detection (LOD)
– Limit of Quantitation (LOQ)
– Reproducibility,
– Selectivity,
– Sensitivity,
– Recovery, and
– Comparison to the appropriate specification or method requirements (e.g. spectroscopic or chromatographic identification).
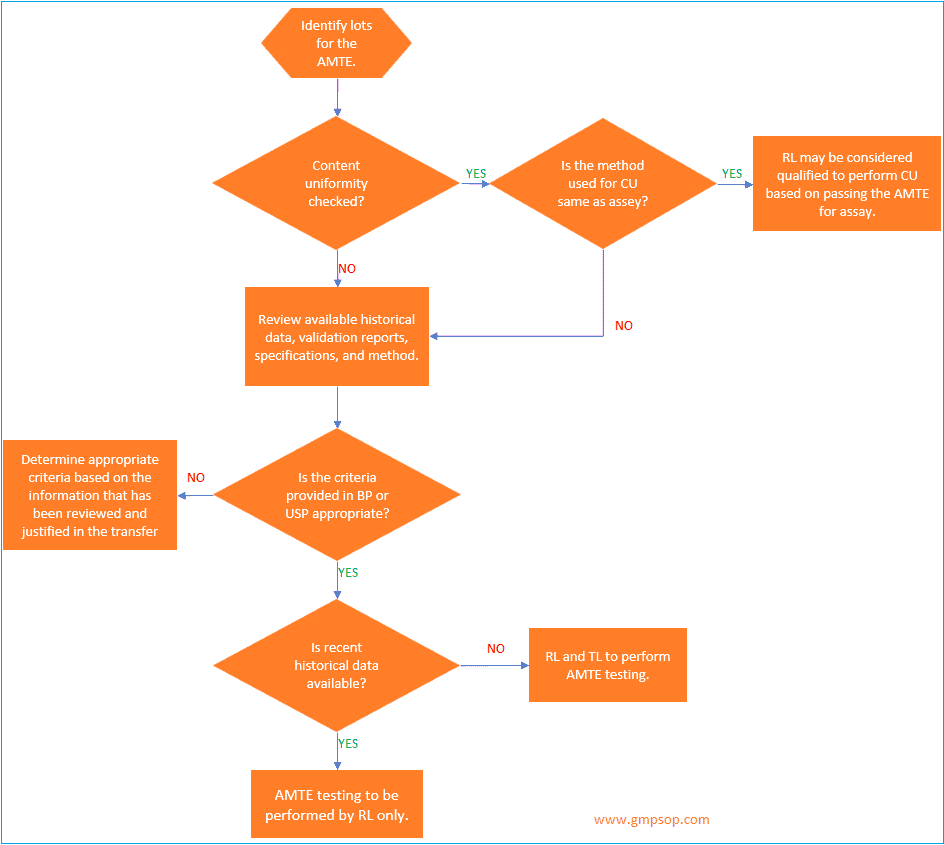
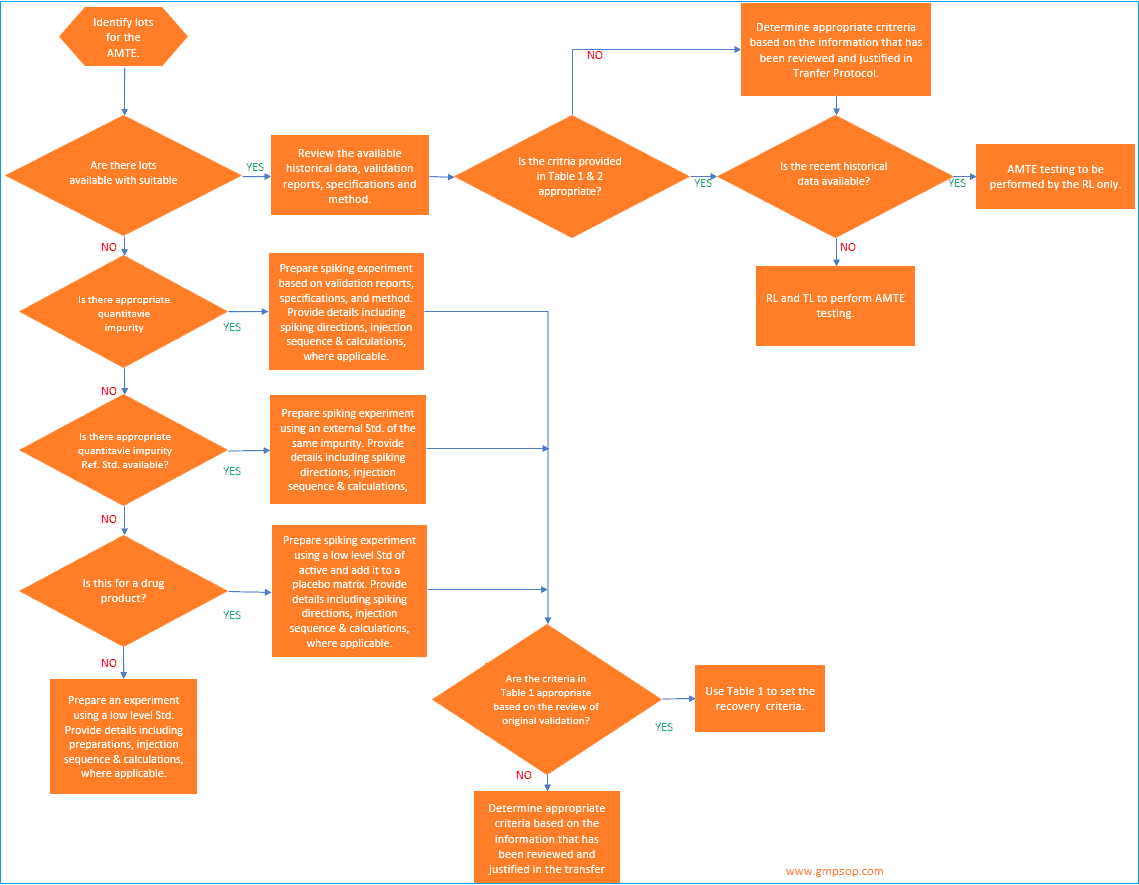
a. How to identify lots to be used for analytical method transfer?
When an active pharmaceutical ingredient (API) or a single strength of a medicinal product is being transferred, a minimum of one lot may be used for the analytical method transfer exercises.
If a single lot is to be used, it is recommended that the testing pattern include multiple analysts,
multiple days, and /or multiple instruments to assess the receiving laboratory’s (RL) ability to generate consistent, reproducible results.
Where multiple lots or batches are used for the transfer, the transferring laboratory (TL) should evaluate the necessity for including multiple analysts, multiple days, and /or multiple instruments in the transfer testing pattern.
If multiple medicinal product strengths are manufactured from a common or similar blend, only the highest and lowest bracketing strengths need to be included in the AMTE.
Successful transfer of the high and low dosage strengths will qualify the RL to test all of the strengths within the bracketed range, provided that the sample preparations for the different strengths are similar.
When identifying the materials to be used for the transfer, the TL will determine if historical data will be used or if comparative data will be generated specifically for the transfer.
Historical data is defined as data generated by a qualified laboratory outside the transfer process.
Some sources of historical data are stability, certificate of analysis, and validation data.
If commercial lots are used for the transfer, it is recommended to avoid using lots whose most recent results lie near the specification limit.
This is not a concern for expired, purposely degraded, or development lots.
b. How to establish acceptance criteria for analytical method transfer?
When setting the acceptance criteria for any method transfer, it is recommended that the validation documents, the specification limits, and any available stability data be reviewed.
The review of stability data, if available, from the lot to be used in the transfer as well as from other lots is important as it may indicate sources of variability other than the method (e.g., product variability) that must be accounted for when establishing the replicate pattern and acceptance criteria.
At the conclusion of the testing, the transferring laboratory should review the data to ensure that the results not only meet the criteria set in the transfer protocol, but should also ensure that there is not a significant bias in the data (e.g., the receiving laboratory passes but their results are consistently higher, or lower, and at the criteria limit).
If a bias does present itself, it is strongly recommended that an investigation be conducted to ensure that potential long-term issues are minimized.
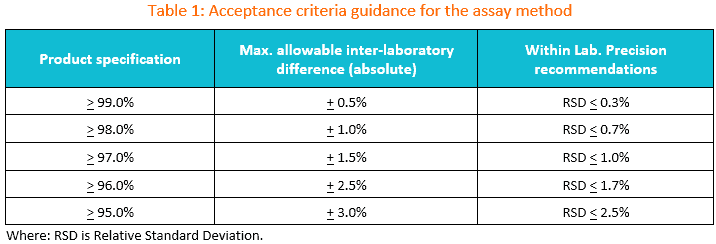
1. Suggested transfer acceptance criteria for the assay method.
You may use the table below as the initial guidance for establishing acceptance criteria during assay method transfer.
If the specification is between two limits in the table, the wider criteria of the two should be used.
The maximum allowable difference between laboratories should be set based on method precision, product variability, and any available historical data.
As always, the criteria suggested in this table should be evaluated against historical and/or validation data as well as the intended use of the method to ensure that criteria are appropriate.
If the criteria to be used are different from those supplied in the table, then appropriate justification should be included in the transfer protocol.
If commercial lots are used for the transfer, it is recommended to avoid using lots whose recent results lie near the specification limit.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
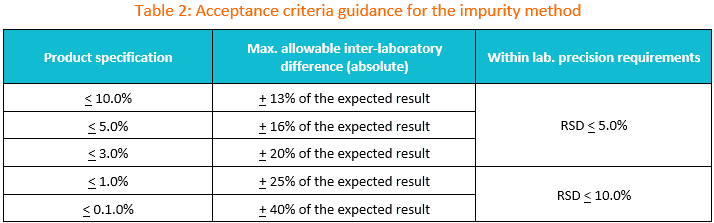
2. Suggested transfer acceptance criteria for the impurity method.
You may use the table below as the initial guidance for establishing acceptance criteria during impurity test method transfer.
If the specification is between two limits in the table, the wider criteria of the two should be used. The maximum allowable difference between laboratories should be set based on method precision, product variability and any historical data available.
If the criteria to be used are different than those supplied in Table 1, then appropriate justification should be included in the transfer protocol.
Reminder: If commercial lots are used for the transfer, it is recommended to avoid using lots whose most recent results lie near the specification limit.
How to use Table 2?
In instances, where the amount of an impurity is less than the specification limit, calculate the inter-laboratory criteria using the table below.
For example, if a lot of material to be used in the transfer has an impurity present at a level of 1.2% and the specification limit is < 2.0%, then the inter-laboratory agreement may be set at + 0.4% (20% of 2.0%).
This criterion should be evaluated against any historical and/or validation data available to ensure it is appropriate.
How to determine replicates and acceptance criteria for different test methods?
a. Replicates and criteria setting when transferring assay methods
i. How many replicates are required?
When conducting assay testing for a composite sample of a medicinal product, assay testing for an API, or unit dose testing of reconstituted lyophilized and liquid forms, it is advisable to analyze a minimum of 6 individual sample preparations per lot.
For unit dose testing of point-of-sale formulations, solid oral medicinal products, transdermal patches, and inhalations packaged in pre-metered dosage units, it is recommended to analyze a minimum of 10 individual sample preparations or lots.
If the content uniformity method follows the same procedure as described in the assay, a site can qualify for content uniformity testing by successfully transferring the assay method.
This justification should be documented during the transfer process.
However, if the Content Uniformity test differs from the assay, it is necessary to analyze 10 samples per lot.
ii. Inter and intra-laboratory acceptance criteria
When transferring methods for assessing the strength of an active pharmaceutical ingredient (API) or medicinal product, the agreement between laboratories can be evaluated by establishing a criterion to determine the absolute or relative difference in overall means, or by employing statistical analysis.
If the approach of absolute or relative difference is chosen, Table 1 can serve as a useful reference.
Additionally, it is important to examine the available validation and stability data for different lots.
If the variability from one-time point to another for the selected lot or other stability lots exceeds the provided guidance or the results obtained during validation, then the acceptance criteria for inter-laboratory agreement may be adjusted accordingly.
When determining acceptance criteria for counter ions and/or preservative content, it is possible to apply less stringent criteria.
Table 1 presents suggested %RSD (relative standard deviation) criteria based on specification limits.
It is advisable to review historical and validation data, when available, to confirm that the method can achieve this level of precision.
If the validation and historical data support it, adjustments to the %RSD can be made, and the rationale should be justified in the transfer protocol.
If the same analytical procedure is used to determine assay and content uniformity, then the transfer protocol should reflect that if the RL meets the criteria for assay, then the RL will also be qualified to perform Content Uniformity.
If two separate methods are used, then both the assay method and content uniformity methods should be transferred.
For content uniformity testing, the results should be evaluated vs. the requirements in the appropriate compendia and compared to the results generated by the TL.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
b. Replicate and criteria setting when transferring impurity methods
Where lots are not available with impurities at a level suitable for meaningful interlaboratory comparison, strategies will be considered to adequately assess the ability of the receiving laboratory (RL) to detect and quantify impurities.
This may include spiking/recovery experiments, where appropriate.
If a spiking experiment is conducted, and at least one specified impurity is available, recovery should be assessed at or between the quantification limit (QL) and specification limit.
For multiple-strength medicinal products made from a common or similar blend, only one dosage strength needs to be spiked.
If the historical data shows levels of an impurity in the lot identified for the transfer below the QL but above the limit of detection (LOD), then it is recommended that the experimental protocol include a requirement to perform multiple analyses (e.g. 3) of an un-spiked sample from the same lot and then use the average results from the un-spiked sample in the recovery calculations to correct for the presence of the impurity in the sample.
In instances where impurities are not available, it may be acceptable to dilute a standard of the main component down to the QL and perform a study to ensure that the RL can achieve the appropriate levels of precision and sensitivity.
Other strategies may be used if they are detailed in the transfer protocol.
i. How many replicates are required?
If the samples contain impurities at levels suitable for inter-laboratory comparison, a minimum of 3 sample preparations/lot should be analyzed when conducting the method transfer.
If the impurity method evaluates the main band assay and impurities from the same sample injection or from a diluted sample, then the assay replicate pattern should be used for both.
For thin-layer chromatography (TLC) methods, only one sample replicate is required.
ii. Inter and intra-laboratory acceptance criteria
If the lot or lots contain impurities at levels suitable for meaningful inter-laboratory comparison, then the guidance provided in Table 2 should be reviewed and applied as appropriate.
Where spiking experiments are required, the following guidance, in conjunction with available validation data, may be used to establish recovery ranges and precision requirements.
For spiking levels between 0.1% and 1.0%, the recommended mean recovery should be 100% ± 25%.
For levels at or below 0.1%, the mean recovery should be 100% ± 40%.
Typically, precision should be set accordingly using either %RSD or the absolute difference between the highest and lowest recovery results.
Where the difference between the highest and lowest recovery results is used, set the criteria at 1/4th of the recovery range (e.g., ≤ 20% High -Low for a recovery range of 140% -60%).
For the transfer of TLC methods, there is no intra-laboratory precision requirement.
When conducting an inter-laboratory comparison, the criteria should be set such that the reported level of each impurity is consistent with the historical TLC result.
If a spiking experiment is to be conducted for a medicinal product, the impurity should be spiked at a level below and above the specification limit.
For active pharmaceutical ingredients, it may be possible to qualify an RL based on its ability to observe the lowest-level standard band.
It is also important to obtain a copy of the RL’s TLC plate for verification of the reported result.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
c. Replicate and criteria setting when transferring residual solvent methods
Even when lots are available with residual solvent levels at or above the QL, it is recommended that a spiking experiment be conducted to accurately assess the RL’s ability to perform the testing.
When conducting the experiment, a minimum of one solvent needs to be assessed to qualify the RL.
Ideally, the solvent with the lowest specification limit should be used. If the historical data show levels of that solvent in the lot identified for the transfer, then it is recommended that the experimental protocol includes a requirement to perform multiple analyses (e.g., 3) of an un-spiked sample from the same lot and then use the average results from the un-spiked sample in the recovery calculations to correct for the presence of the solvent in the sample.
i. How many replicates are required?
At a minimum, three spiked samples should be analyzed when transferring a residual solvent method.
ii. Inter-and intra-laboratory acceptance criteria
The following guidance, in conjunction with available validation data, may be used to establish recovery ranges and precision requirements.
For spiking levels between 0.1% and 1.0%, the recommended mean recovery should be 100% ± 25%.
Typically, precision should be set accordingly using either %RSD or the absolute difference between the highest and lowest recovery results.
Where the difference between the highest and lowest recovery results is used, set the criteria at 1/4th of the recovery range (e.g. ≤ 20% High-Low for a recovery range of 140-60%).
d. Replicate and criteria setting when transferring identification methods
i. How many replicates are required?
When transferring an identity method, single sample preparation may be used for the assessment. However, it is also possible to include multiple samples, including those that would fail the test.
ii. Inter-and intra-Laboratory acceptance criteria
Typically, identification methods have acceptance criteria within the method itself. Therefore, it is acceptable to qualify for the RL by meeting this requirement.
No inter-or intra-laboratory acceptance criteria are required. However, if the identity method involves an intricate technique (e.g. proteolytic map identities) it may be advisable to include one intra-laboratory criterion to ensure the RL can perform the test consistently.
e. Replicate and criteria setting when transferring dissolution methods
i. How many replicates are required?
At a minimum, 12 units/lot are required to transfer a dissolution method.
If a review of historical data for the lots to be used in the method transfer, as well as other lots, shows product variability > 10% RSD, then it is recommended that the RL test 2 x the minimum requirement for the specific dosage.
ii. Inter and intra-laboratory acceptance criteria
In general, each individual result must meet compendia requirements.
Regardless of formulation, the interlaboratory acceptance criteria should be set using the average results at the final test time indicated in the product’s specifications.
A typical inter-laboratory acceptance criterion is ± 6% (absolute) based on a long-term precision (RSD) of 10%.
However, historical data such as stability data from multiple time points, should be reviewed.
If the data supports another criterion (e.g. time point to time point differences are >6%), then the more appropriate criterion should be used with suitable justification provided in the transfer protocol.
An extended-release formulation will have multiple test time points and the medicinal release ranges are listed in the specification for the earlier test times.
In these cases, it is difficult to set inter and intra-laboratory acceptance criteria. It is possible to set a criterion that the RL results must be within the ranges provided in the specification.
It is strongly recommended that the data be reviewed and compared to the TL data to ensure there are no biases evident in dissolution testing.
If there is a suspected bias in the data, further investigation is warranted.
f. Replicate and criteria setting when transferring particle size (non-sieve) methods
i. How many replicates are required?
It is recommended that at least 6 samples be analyzed for a given lot.
ii. Inter-and Intra-Laboratory Acceptance Criteria
If validation data is available, it may be used to establish appropriate inter and intra-laboratory requirements.
Typically, the inter-laboratory acceptance criteria should be < 10% relative difference between the TL and RL at each specification point. RSDs should also be < 10%.
Conclusion
It is a GMP expectation that when one validated test method is transferred from one laboratory to another, certain experiments need to be carried out methodically to prove that the transfer did not impact the validation attributes of the method.
That means for the same set of samples but different testing environments, the method should produce equal and unbiased results pre- and post-transfer.
The process is commonly known as analytical method transfer in GMP.
Both transferring and receiving laboratories have specific responsibilities to meet in the method transfer process.
Comparative studies are the most common approach to method transfer where the same set of samples are collected from the same lots and tested in both transferring and receiving laboratories simultaneously using the same method.
The results are then compared against pre-determined acceptance criteria to evaluate the closeness of accuracy, precision, and robustness of the method.
The laboratories need to design a method transfer protocol in cooperation that should outline the transfer process, responsibilities, validation history, acceptance criteria, employee training, etc.
Successful method transfer requires a certification to be provided by the transferring laboratory to receiving laboratory.
All documentation and records generated during the transfer process must be archived for future audits.
Please leave your valuable comment below on what you think about the process we have detailed in this article. Don’t hesitate to raise questions and clarifications where appropriate.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.