What is cross contamination in pharmaceutical industry
- Kazi
- Last modified: March 13, 2025
What’s cross-contamination in pharmaceutical industry?
You should not be unfamiliar with it if you know how pharmaceutical facilities operate. Cross contamination in pharmaceutical industry is a serious issue which can affect the quality and safety of drugs.
To prevent cross-contamination, pharmaceutical companies implement strict hygiene and sanitation procedures, robust cleaning programs, use dedicated equipment & facilities, and perform regular quality control tests and QA release activities throughout the supply chain.
We will dive deep into how cross contaminations are prevented in the pharmaceutical industry later.
Cross contamination in the pharmaceutical industry can be described as an accidental inclusion of product of another batch or unknown foreign material into a finished batch, which was not intended or not mentioned on the label.
Put this differently: cross contamination may occur by transferring substances, microorganisms, or other unwanted material from one product to another, leading to contamination of the final product.
Cross contamination is dangerous in pharmaceutical industry as it can severely compromise patient safety if the content is not pure and the product package differs from what is written on the label.
Table of Contents
Key Takeaways
- Cross-contamination in the pharmaceutical industry occurs when unintended substances, such as particles from other products, microorganisms, or chemicals, are introduced into a new drug batch during manufacturing.
- Impurities in manufacturing ingredients can be a source of cross-contamination. Raw materials, including active pharmaceutical ingredients (APIs) and excipients, can introduce contaminants.
- Materials that are not adequately sterilized or stored can introduce microorganisms, leading to microbial contamination.
- Residue from previous batches can result in cross-contamination. If manufacturing equipment isn't thoroughly cleaned between production runs, residues from previous batches can contaminate new products.
- Some large and complex equipment and pipework have hard-to-clean areas. These are prone to creating condensate, which can trap particles and may cause contamination.
- If personnel do not follow hygiene practices, they can introduce contamination into pharmaceutical products. Workers who do not follow strict hygiene protocols can transfer contaminants through their hands, clothing, or personal items.
- Personnel moving between production zones without proper gowning and PPE can spread contaminants in different production zones.
- Airborne particulates such as dust, aerosols, microorganisms, or other particles can settle on products, leading to contamination.
- Inadequate cleaning of floors, walls, and surfaces can allow contaminants to accumulate and spread.
- You can minimise the risk of cross-contamination by introducing comprehensive training and education of your personnel. Regular training programs ensure that staff understand contamination risks and adhere to hygiene protocols.
- Using dedicated equipment exclusively for a single product or product type minimizes cross-contamination risks. Clear labeling of equipment is essential to prevent accidental misuse of dirty equipment.
- To prevent contamination, clean your equipment regularly with validated cleaning procedures. Ensure that all residues are removed or brought down to an acceptable limit before using them to manufacture a new batch.
- Routine checks and maintenance of equipment can ensure optimal operation and cleanliness.
- Before introducing raw materials into the processing, conduct quality control tests routinely to ensure the ingredients do not contaminate batches with impurities.
- Use pressure gradients and HEPA filters in air-handling units to control airflow between graded zones. Appropriate air pressure differentials can prevent airborne contamination in the manufacturing areas.
- Design your facilities with smooth surfaces and minimal ledges, and ensure proper airflow to reduce contamination risks.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Examples of cross contamination in pharmaceutical industry?
If you have worked in a tablet filling line, you may have occasionally found fragments of tablets that are of different sizes, shapes, colors, and active ingredients under the vibrating tablet feeder tray.
If the tablet feeder area is not cleaned regularly, sometime after each batch of filling, one of those rogue products can cause serious contamination to the next batch.
Production line Operators have to wear special gowns, head covers, gloves and masks to prevent potential cross contamination in the pharmaceutical products.
You might have seen pest control baits, zappers, and other mechanisms placed all around the production and warehouse facilities.
These are implemented and monitored regularly to find pests such as rodents, insects, etc., and ultimately to prevent cross contaminations of finished goods and raw materials.
Quality control analysts are busy testing related substances, impurities, degradation products, or environmental monitoring tests for microbial contamination.
Pharmaceutical businesses must take many more tests, cleaning and other preventions to ensure their products are free from unwanted cross contaminations.
Separation of production lines, warehouse storage areas, and material or personnel flows are strictly controlled for the prevention of cross contaminations.
You might have seen Validation Engineers are busy validating cleaning methods to be used in production vessels, transfer lines, filling lines, processing equipment, or sterilization cycles.
All for preventing potential cross-contamination from carry-over of chemicals from one product to the next.
Since cross contamination can happen during the whole manufacturing chain, i.e. at the supplier facility, during storage, and processing and packaging steps, there is a need for a comprehensive analysis of cross contamination risks.
Cross contamination data that can make you nervous!
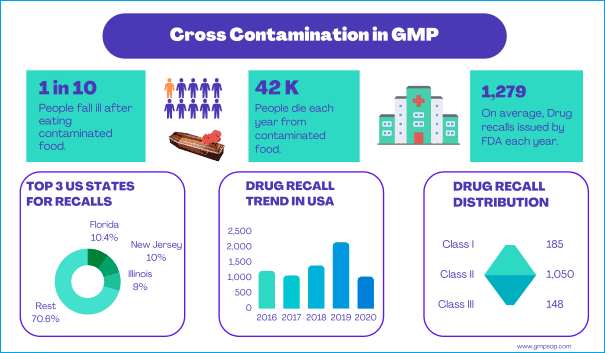
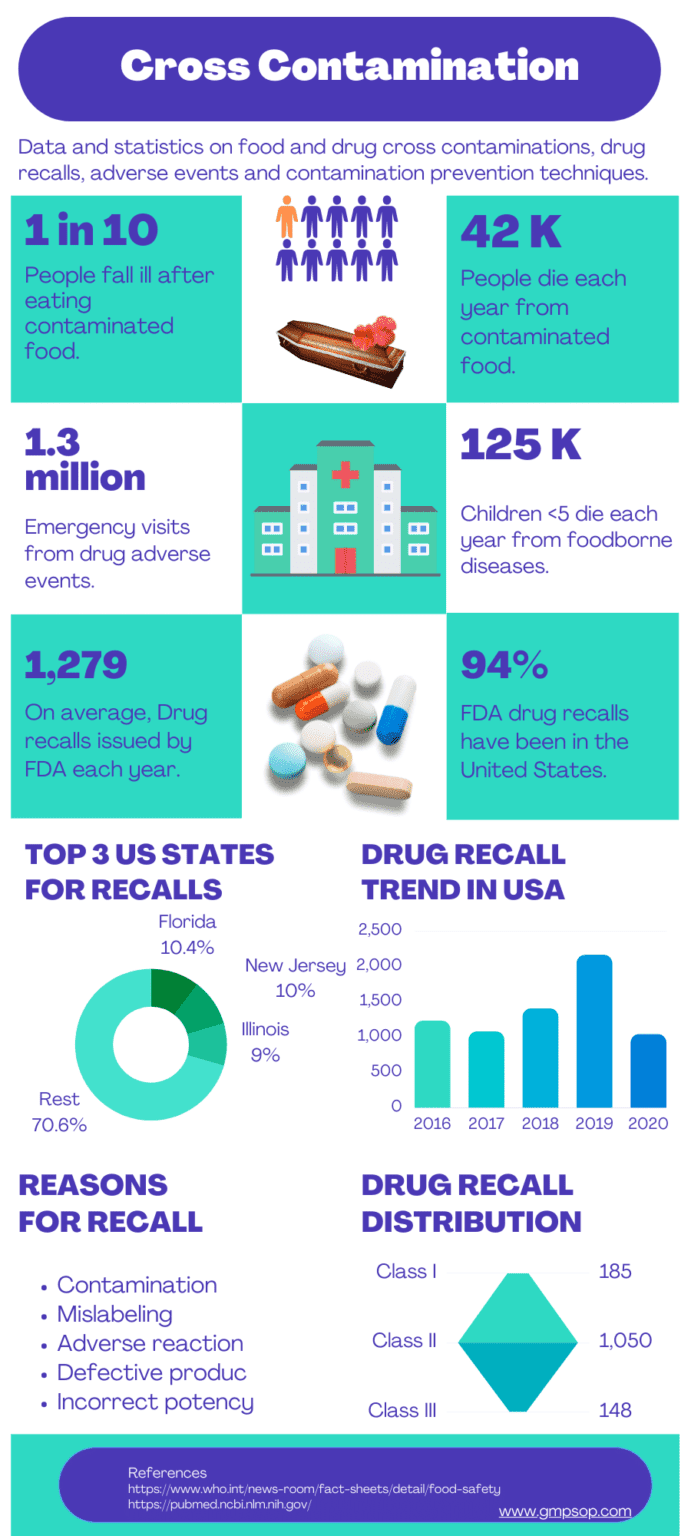
– According to WHO report on 20th April 2020, 1 in 10 people fall ill after eating contaminated food worldwide.
– 420,000 people die every year from contaminated food, which is a combined loss of 33 million healthy life years.
– Children under five years carry 40% of the foodborne disease burden. Responsible for 125,000 deaths every year.
– According to the CDC, adverse drug events cause around 1.3 million emergency department visits per year. About 350,000 of those patients need to be hospitalized.
– On average, the FDA has issued 1,279 drug recalls every year. Many of those are due to cross contaminations.
– In 2019, the FDA publicly reprimanded 21 companies for cross-contamination. 94% of FDA drug recalls have been in the United States.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
What are the common sources of cross contamination?
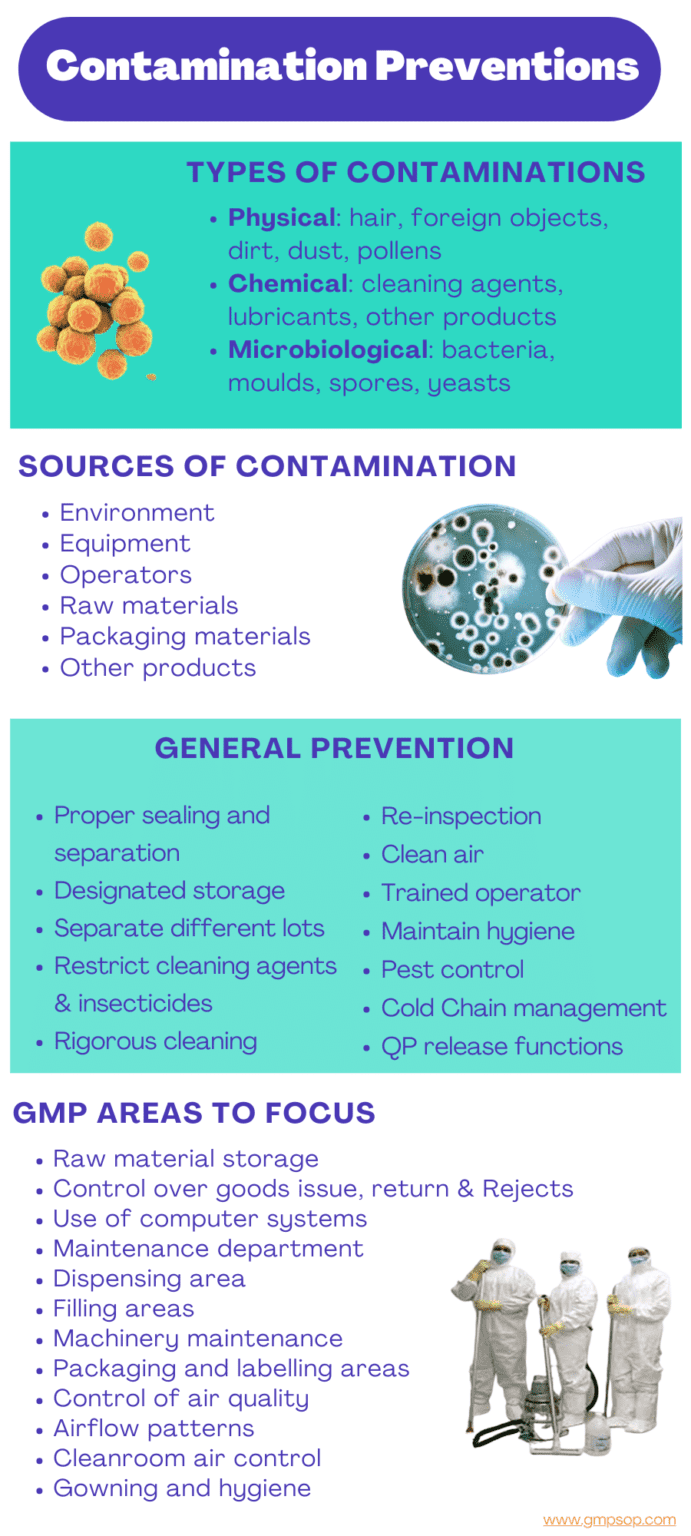
Cross contamination in the pharmaceutical industry can occur from various sources such as poor hygiene practices, improper equipment cleaning, shared production facilities, etc.
Cross-contamination of products can be caused by one or more of the following ways:
a. Contamination of a batch with a previous batch of the same product
b. Contamination with a different product through carryover or proximity of production lines
c. Contamination by a foreign starting material, usually of the dispensary or in the store
To be clear, we have listed a few commonly found sources which are frequently responsible to cause cross contamination of pharmaceutical products.
1. Raw materials: Cross contamination can occur from impurities in raw materials, such as active ingredients, excipients or microorganisms or pest infestation of food grade ingredients.
2. Equipment: Manufacturing equipment can be a source of cross contamination if not properly cleaned between batches. This can result in residue from previous batches contaminating subsequent batches.
Validated cleaning methods are important to eliminate the possibility of such cross contamination.
3. Personnel: Personnel handling the products can be a source of cross contamination if they are not wearing proper protective clothing or if they do not follow good hygiene practices.
Appropriate gowning proportionate to the processing environment or zones can reduce personnel-induced cross-contamination.
4. Environment: The manufacturing environment can also be a source of cross contamination, as air, surfaces, and water can carry microorganisms and impurities.
5. Cross-contact: Cross-contact can occur when products are not properly separated during storage or handling, leading to the transfer of impurities or active ingredients from one product to another.
How to prevent cross contamination in pharmaceutical?
a. Preventing cross contamination through personnel?
1. Personnel training
All personnel whose duties take them into or impact processing work within production, laboratories, and storage areas must be provided training on cross-contamination risks and prevention.
Newly recruited personnel must understand possible cross contamination risks and the consequences of contamination.
Annual GMP training programs should include elements of cross contamination prevention. Training must be documented on individual training records showing the date and scope per subject.
Personnel is responsible for line-clearance procedures must be specially trained and authorized for this task.
2. External visitors
Please don’t let external visitors enter production areas. If this is unavoidable, they should be given information in advance not to touch materials or products and stay within assigned areas.
Visitors must be closely supervised and never left unattended.
Where products are being handled, all visits within production must be documented giving time, date and name of visitor.
External personnel, like maintenance people, consultants, and service personnel, must be given the same information as visitors and be adequately supervised.
3. Personnel clothing
All personnel in areas where single units of products are handled must wear protective clothing without pockets.
A small pocket for necessary items like pens and reading glasses is allowed.
Personal medications must not be brought into production areas.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
b. Controling facilities to prevent cross contamination?
1. General facilities
Maintain premises carefully, ensuring that repair and maintenance operations do not present any risk for cross contaminations.
Sufficient space must be available to avoid storage of products in corridors designed for communication only.
Steps must be taken to prevent the entry of unauthorised people into production, storage and quality control areas. Separate air-locks should be used for personnel and material entering classified areas.
Illumination must be appropriate, particularly where visual identification is carried out, such as online inspection and label control.
Crossing flows of unlabelled products, unsterilized/ sterilized products, and uncleaned/cleaned materials must be avoided.
2. Production Area
Premises should be laid out in such a way as to allow the production to take place in a logical order to minimize intermediate storage of unlabelled products.
The adequacy of the working and in-process storage space must permit the orderly and logical positioning of products and materials to avoid cross contaminations.
Premises for the product packaging areas must be specifically designed and laid out to reduce cross contamination risks.
Perform all operations with unlabelled products within a separate area, which excludes unauthorised access.
3. Storage Areas
Quarantine areas must be clearly marked, and access should be restricted to authorized personnel. Any system replacing the physical quarantine must give equivalent security.
Keep only one material or required materials issued for one batch on one pallet unless clear physical partitioning exists.
Pay particular attention to printed materials. They must be stored in adequately secured conditions to exclude unauthorized access.
Store all labels and leaflets in a locked room. Unauthorised access to this room must be prevented and the number of authorised persons limited.
A locked or properly supervised area is also required for all material in single pieces of printed primary packaging materials like ampoules, tubes, or cartons. These materials must be kept in closed containers.
Store other printed packaging material, such as blister foils, in areas with limited access for unauthorized personnel.
You can store bulk material in sealed containers in a secure area. Different batches must be separated and marked to prevent cross contamination.
Could you mark and store rejected materials and products in a separate locked area?
You can store the recalled products in a separate and locked area.
Mark and store returned products in the quarantine area before QA re-evaluation.
c. Control of equipment in cross contamination prevention
1. Design and Construction
Equipment that is difficult to clean or has space where products can be concealed can increase cross contamination risks. Consider any risk of potential cross contamination during the design and construction of equipment.
Lines of equipment should be fit together to give an even flow of product without any need for removal of product from the line.
For line clearance, any protective screens must be easily removed.
2. Used Equipment
Used equipment must be emptied of product and printed material before being brought outside the cleared area.
Such equipment must also be separated from any cleaned equipment.
Pay special attention to equipment not always dedicated to a special line such as metal-checkers, de-dusters, in-process control equipment, boxes, cages, containers, etc.
Such equipment must not be brought into an area unless cleared as directed under the Line clearance procedure.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
d. How to control production operations in preventing cross-contamination?
1. General controls
Before any processing operation starts, please make sure that the work area and equipment are clean and free from any starting materials, products, product residues, or documents not required for the current operation.
Before the packaging operation begins, please make sure that the work area, packaging lines, printing machines, and other equipment are clean and free from any products, materials, or documents not required for the current operation.
Could you perform line clearance according to an appropriate checklist?
Operations on different products should not be carried out simultaneously in the same room without adequate separation.
At all times during processing, all materials, bulk containers, major items of equipment, and, where appropriate, rooms used should be labeled or otherwise identified with an indication of the product or material being processed, its strength (where applicable), and batch number.
Where applicable, this indication should also mention the stage of production.
Filling and sealing should be followed as quickly as possible after labeling. If this isn’t the case, please follow the appropriate procedures to ensure no cross contaminations or mislabelling can happen.
Implement checks on yields and reconciliation of quantities. These should be carried out as needed to make sure there are no discrepancies outside acceptable limits.
2. Set-up of Equipment and Lines
Remove all previously used materials or manufactured products before equipment or arrange a line for a new operation.
Line clearance must ensure that any materials used for set-up have been removed and reconciled.
If a set-up operation is performed after line clearance, any material used must not give rise to cross contamination risks.
3. Use of “Dummies” and Calibration Samples
Dummies are tools to run through a complicated line to ensure no product has been left on the line. Thus, they can be used where appropriate.
Dummies or calibration samples used for checking scales or inspection machines must be used as follows:
– They must be well identifiable from the real product and reconciled after use.
– Dummies and calibration samples must be kept segregated from the line and never be kept above the product flow.
– The correct number should be checked for cleaning and line clearance reconciliation.
e. Control of starting materials in cross contamination prevention
1. Purchase of starting material
Purchase of packaging and labeling materials should only be made from approved suppliers, which can ensure the correct identity of the materials.
2. Internal Labeling
Before released to the store, internal labels are to be put on the material by an authorised person and checked by a second person or QA staff, unless electronic identification is used.
Initial identification of the material from the supplier should not be covered.
3. Sampling
When a container is opened for sampling, it must be resealed to the same security as beforehand.
4. Internal Labelling Standards
The standard for the labeling of starting material and primary as well as printed packaging materials must meet the following basic requirements:
There must be an internal identification system.
A material must be identified by a company’s adopted name or a code.
Furthermore, a machine-readable code can be used. Other identification except that of the last supplier should be crossed over.
Labels should be machine-printed and have good legibility.
Two authorized individuals must fix labels and check for correct identity and corresponding labeling unless electronic identification is used.
The second individual who checks the correct identity and labeling must verify this by signing.
Primary labeling should be placed on the material container, not lids or wrappings.
Any delivery to production of several units of a batch/lot that does not carry internal labels must be kept together and followed by at least one internal label.
5. Delivery to Production
Where simultaneous dispensing of different materials is performed, the operations must be physically segregated.
Two individuals must verify the identity of dispensed material unless electronic identification is used. All materials for a batch should be kept together.
Each dispensed package of starting material must be labeled with the batch number for the intended batch and material identity and quantity.
The material must be held in a dedicated area if not delivered directly to production.
Provisions must be made to exclude all risks for cross contamination of packages for different batches.
Using closed containers, trolleys, or a covering net is recommended.
6. Use of starting material
Before use, the correct identity of all starting materials for the batch should be checked by two individuals or verified by an electronic system.
f. How to control material flow to prevent cross contamination?
1. Interruption in Production
Design the production area so that products are made and labeled with as few interruptions as possible.
Whenever a production chain is broken, the bulk material must be stored in secure conditions to exclude unauthorized access to the material.
When an unlabelled bulk product is introduced into a sequential manufacturing step, identity must be verified by two individuals.
Automatic (electronic) identification systems are encouraged. If the latter are used, a second check is not required.
2. Return of Material from Production
Please don’t forget to return dispensed excess materials where possible.
Could you destroy the excess batch-coded material?
Other excess material should normally be rejected. Otherwise, it must be returned to the appropriate store with proper controls.
It is not permitted to store surplus material in the production areas.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
g. Preventing cross contamination in packaging operations
1. General controls
Packaging operation provides the greatest risks for cross contaminations. Therefore, follow these three principles:
– Personnel must be selected and trained for the task,
– Packaging of different products must be physically segregated,
– There must be an effective program for line clearance of the work area, packaging lines, printing machines, etc.
2. Material Handling
You can only request starting material quantities required for the batch from the store.
Identity of current material must be consistent with packaging Instructions, batch records, materials list. Article numbers must be used to verify identity.
When different names are used for the same material (article number), all personnel must know it (e.g., when generic names are used in parallel with trade names).
The name and the batch number of the product being handled must be displayed so that each person authorized to enter the area will be fully aware of the current product being handled.
The name of the product should conform with the label of the product.
3. Line Flow
Filling and sealing must be followed as quickly as possible after labeling. Where this is not the case, the following procedures must be applied.
Unlabelled units must be collected in containers, closed, sealed, and stored in designated storage areas.
Please verify the identity of each container before labeling.
Unplanned, temporary production stops with the consequence that filled but unlabelled products have to be removed from the line, or normal product flow is to be handled in the following manner.
Such material must be collected in containers or boxes that have undergone normal line clearance operations and checked to ensure they are empty.
When not in use, such boxes must be stored upside down.
The boxes must not be left near the line (i.e., the area cleared during line clearance).
Before material is returned to the line, approval should be given by QA/QC or Production Management.
Two individuals must check their identity, and the event must be recorded in the batch records.
Material that has been rejected by checking stations must be handled as follows.
The material must be collected in cages or boxes through which inspection is possible (net, glass, or acrylic).
A procedure must be in place that describes the conditions under which recovery is permitted.
Two individuals must make any subsequent return of product or packaging material to the line. They must separately check the material for correctness and identity.
If material is added to the line upstream of the station that initially rejected it, two people are not required.
If the material has left the line for inspection purposes and has been transferred to a checking station, it may be returned if checked by a second person for correct identity.
If a product is picked up for visual appearance inspection only, it may be returned if done immediately.
Return is strictly prohibited if the material has been transferred outside the area cleared by line clearance.
Material that has fallen off the line or is found outside the normal product flow of a line must be rejected immediately and discarded.
4. Labelling
Avoid relabelling poorly labeled containers.
If necessary, the rules under Line Flow (above) will need to be strictly followed.
This means a second person must check that return to the line or inclusion in the batch.
Manual relabelling should be avoided and only justified if labels are taken from the line after code-reading and imprinting.
The same principles as above should be adopted when boxes or similar secondary identification are emptied and changed.
5. Verification of Printed Packaging Materials
If pre-printed primary containers are used, they must be identified as close to the filling operation. Automatic systems for verification of correct identity should be used.
6. Temporary Stops
The area must be locked to exclude unauthorized access if a line with the product or printed packaging materials is left unattended.
If any unforeseen interruption lasted more than fifteen minutes for technical reasons, the incident must be reported, e.g., in the batch records.
During longer breaks, e.g., during nights, empty the line from the product, but printed packaging material can be left in their positions.
The room must be locked or secured to prevent unauthorized entry.
7. Returns
Batch-coded materials must be destroyed and the destroyed quantity recorded. Other materials must be handled as directed in Section F, Material Flow.
8. Records
There must be a log for each line where the following must be recorded:
– Product identity, size, etc.
– Batch No,
– Start of packaging (day and hour),
– Longer breaks,
– Finish of packaging (day and hour),
– Unforeseen events,
– Maintenance.
h. Control of visual inspections in cross contamination prevention
1. Segregation
Each batch must be kept in a separate room or area limited by partitioning.
I’d like to point out that cleaning and line clearance must be performed as directed in Section J. Line Clearance.
2. Handling
Unlabelled products must be handled in closed and sealed containers outside the inspection area.
Rejected material should be put in containers of such kind that reintroduction into the accepted products is prevented.
Containers for rejected material must be marked as such.
Empty containers into which product is collected must be checked carefully for emptiness and kept upside down.
Such containers must be made in a way and in a material that excludes any possibility that products can remain in the container when inverted.
3. Display of Operation
The name and batch number of the product being inspected must be displayed so that each person authorized to enter the area will be fully aware of the current product being handled.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
i. How do you control rework operations to prevent cross contamination?
1. General controls
Rework means reprocessing, repackaging, relabelling, or any initially unplanned activity to recover or change a product.
Such operations are known to add major risks for cross contaminations and should therefore be avoided or very carefully controlled.
Rework and identifying labeling must comply with the principles of Good Manufacturing Practice.
These include acceptance from authorities of suitable premises and defined and documented procedures.
2. Reprocessing
Reprocessing of products should be exceptional and must be described by approved procedures and allowed by regulations.
3. Repackaging
All operations, including handling printed material, must follow the instructions for Printed Packaging Material in these Standards.
4. Relabelling
Covering the original, primary identification or batch number is a highly unusual event and must be carefully controlled and documented.
Any addition of secondary printed packaging materials, e.g. putting parenteral products in boxes must follow regulations under packaging operations.
j. Line clearance in cross contamination prevention
1. Line clearance
Line clearance is the check that must be made immediately before an operation is started. Line clearance also includes “room clearance.”
Line cleaning is used for all the necessary operations that terminate a process.
Cleaning includes dismantling and washing equipment and removing all products, packaging materials, and documentation.
A cleaning operation must always be finished with a check of the intended result.
2. Instructions and Checklists
Where different batches, products, or printed packaging materials are processed in sequence, cleaning and line clearance must occur before commencing the next operation.
Such operations include mixing, tableting, compounding, filling, leak-testing, packaging, labeling, and imprinting.
Cleaning must be described in written instructions, which must be specific.
The cleaning instructions must include necessary details for proper cleaning of all parts of an area and equipment as well as the items that must be focused on during the check of cleaning.
All material or equipment not needed must be removed from the area.
The instructions for line clearance must detail all checkpoints and how to perform the line clearance operation. Such instructions should include drawings or pictures showing where to check.
It must also point out that tables, desks, etc. must be checked.
Furthermore, the instructions should tell the most efficient way to check and how to dismantle screens and similar objects.
Included in the line clearance instruction must be a specific checklist.
This checklist must be detailed enough to ensure no part of an area, equipment, or line is missed.
3. Authorisation for Line Clearance
Personnel, including supervisors and management who perform line clearance, must be properly trained and authorized for each type of operation or line.
Retraining must occur at prescribed intervals.
Representatives from QA should periodically carry out line clearance or evaluate its efficiency by participating in its performance.
4. Execution of Line Clearance
The following prerequisites must apply to start line clearance:
Previous operations must have been finished, and cleaning must have been checked.
To be cleared, no product or printed packaging material must be present in the area.
Equipment and lines must be dismantled, and machine parts, etc., must have been cleaned. The parts must be present in the area for checking.
The batch record for the operation must be available.
The previous operation must have been recorded in the logbook.
The following items must be made during the line clearance operation:
– Sign the specific checklist for line clearance as directed.
– A second authorized person must repeat the line clearance independently. Thus, two individuals must perform the line clearance.
– Any finding of product or printed packaging materials that should have been removed from the area during the cleaning operation or the first-line clearance check must be reported in writing to plant management and QC.
Records of such reports must be available for internal inspections and corporate audits.
5. Further Information on Line Clearance
Sometimes, it is possible to combine the cleaning check and the first-line clearance check.
This is accepted when there is only a short time lag between two operations and the cleaning does not involve major dismantling and washing operations.
However, the line clearance must also be made by a second person.
There must not be any substantial time lag between line clearance and manufacturing.
It is unacceptable to perform line clearance in the afternoon and start the manufacturing the next morning unless the area has been securely locked and no unauthorized entries have been possible.
Any equipment brought into a cleared area must have been checked using the principles described in this chapter.
This is especially important for repair and maintenance equipment brought in during an operation that is not finished.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
k. Control of printed packaging materials in preventing cross contamination
1. Supply of Printed Packaging Materials
A supplier agreement should state that each material or product code must be kept separate from others.
This is especially important for printed material in single-unit boxes, ampoules, tubes, or leaflets where cross contamination occurs.
Gang-printed materials are not allowed.
Material supply should only be accepted from approved suppliers.
Approval should only be given to suppliers who have a full understanding of the consequences of material cross contamination and are prepared to eliminate cross contamination risks in their production.
Approval should only be given after an audit from the company. An approved supplier must accept periodic audits.
2. Storage of Printed Packaging Materials
Printed packaging materials must be received, quarantined, stored, and delivered in a manner as to exclude unauthorized access to the material.
Any sample of printed material kept as standards must be marked as such and obliterated to prevent normal use.
The samples must never be kept in an area cleared for production.
3. Delivery and Returns of Printed Packaging Materials
For each packaging operation the required materials should be delivered from the store to the packaging department.
Material for a batch should be kept together and segregated from material for other batches. Material must be sent to the packaging department or collected from the store at a designated area.
Any need for more material during an initiated packaging operation must be delivered following normal routines.
It is unacceptable for material to be released from a store during shifts not operated by the store.
Once labeling materials are dispensed to pack a batch, only unopened containers of labeling materials may be returned to the label storage area, except roll labels.
Roll labels may be returned if a documented inspection has been completed to ensure that labels imprinted with variable copies are removed from the roll.
Unused batch-coded packaging materials must be destroyed and the destruction recorded. There must be written procedures for the identification of materials returned to stock.
l. Special requirements for labels and printed primary packaging materials
1. Labels
Roll labels, supplied or printed online, are preferred. Cut labels require electronic or duplicate human verification.
A written agreement must be established with approved suppliers containing, at a minimum, the following provisions:
– Labels must be counted, and the number given on each roll,
– Labels must be code-read. This operation has to be documented and signed on each roll.
– Splices should be avoided as much as possible. If more than one splice per roll is present, evidence of the integrity of each splice must be provided in the batch records.
– Each roll should be wrapped in plastic film before delivery.
Please look over the supplier operations regularly, usually once a year.
A received shipment must immediately be quarantined in the label store.
The label store must be kept in a locked room excluding unauthorised people to enter.
The label store should have sufficient space to permit good order and dedicated areas for quarantine, approved material, rejected material, and a dispatch area.
The label store must be well illuminated and equipped with a counting and a code-reading machine if needed.
There must be an adequate separation between different product labels and batches (lots, editions) of the same label.
Materials from the label store should be delivered in sealed boxes or trolleys.
If an intermediate label store is used in the packaging area, this store must be locked.
For cut labels, there must be an electronic device for conducting a 100 percent identification for correct labels on the packaging line.
The code-reader must be set from a master. Otherwise, a duplicate human inspection must be performed.
Code-readers, label counters, or similar devices must be checked to ensure they operate correctly. These checks should be performed and recorded at least before and after an operation.
If the device on the packaging line breaks down during an operation, work should stop until it is repaired.
The ongoing operation may be finished by having the remaining labels code-read in the label store.
For smaller quantities, a 100 percent manual inspection verified by a second person is acceptable to finish the operation.
Discarded labels from the packaging line must be safely collected (e.g., in a sealed container).
Labels returned to the label store must be delivered in sealed boxes or locked trolleys.
Written procedures must be followed to reconcile labels issued, used, and returned.
Any significant or unusual discrepancy observed during reconciliation of the amount of bulk product and labels and the number of units produced must be investigated and satisfactorily accounted for before release of the batch.
Pre-set, narrow limits based on historical operating data should be used.
In manual packaging, the labels may be code-read in the label store before delivery.
For small quantities of labels, a 100 percent manual inspection verified by a second person during the packaging operation is acceptable.
On-line printed labels must be produced by a validated system assuring correct identity, batch number, etc.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
m. Control of pre-printed tubes, ampoules, vials, bottles, and bags
Handling pre-printed containers must be given special attention due to cross contamination risks.
Just to let you know, the same rules are valid for labels.
Store the material in segregated areas, which should be locked or kept under constant supervision by authorized personnel.
The material must be stored, delivered, and returned to stock in closed and preferably sealed containers.
Pre-printed material must be automatically identified on the production line. If this is not performed, a 100 percent examination of identity must be conducted on each finished product container by an operator.
The number of units produced in each major production step must be reconciled using the same principle for labels.
n. Special requirements for printed secondary Packaging materials
Cartons and boxes should be code-read on the packaging line. Verification by manual means requires a duplicate inspection.
An edge code may be used as an alternative for inserts and leaflets. Personnel must be trained in the correct use of edge-code identification.
o. Special requirements for leaflets (inserts)
I think leaflets on rolls should preferably be used and handled like previously given for labels.
Cut leaflets must be given special attention due to cross contamination risks. In addition to the requirements for labels, the following requirements also apply:
Cut leaflets must be stored, delivered, and returned in a closed container.
Cut leaflets must be code-read and have an edge code to facilitate visual inspection.
p. Miscellaneous controls in preventing cross contamination
1. Sampling
Samples taken away from an operation should not be returned.
Sample containers for products must have an appearance and size different from the packaging material under process.
Sample containers should not be labeled with labels used for the finished product.
2. Use of Compressed Air
Guns with compressed air are sometimes used for cleaning packaging lines.
However, it is an obvious risk that tablets, labels etc. can be transferred unforeseen and hidden at places not included in the normal line-clearance checklist.
Compressed airguns should be used with special attention to the risks and with appropriate restrictions.
When compressed air is used for cleaning, special attention must be devoted to the possibility of transferring products to places above the product flow. These places must be included in the line clearance.
Compressed airguns or similar devices are prohibited after the line clearance.
Conclusion
Next time if this question “What’s cross contamination” crosses your mind and overwhelms you, think it is responsible for causing serious harm to human health if not controlled at the root.
In the pharmaceutical industry, cross contamination can affect the quality and safety of drugs, leading to adverse effects for patients.
To prevent cross contamination, it is essential to follow strict hygiene and sanitation procedures, use dedicated equipment and facilities, and perform regular quality control tests.
Risks of cross-contamination may arise from starting materials (including water, from the environment, uncontrolled release of dust, gases, vapors, sprays or organisms from materials or products in process.
Sources of cross contamination also include residues from uncleaned equipment, untrained personnel, and their clothing.
From Cross-contact when starting material, packaging materials and products are not adequately separated during storage or handling, leading to transfer of impurities or active ingredients from one product to another.
In the pharmaceutical industry, you must take numerous preventive measures to reduce or eliminate cross contamination risks.
A summary of those can be listed as follows:
a. Maintain proper sealing, separation, and storage of raw materials.
b. Take adequate care in managing the dispensary to exclude the opening of different lots of containers in close proximity.
c. Employ thorough and rigorous cleaning of all equipment, utensils, transfer lines, extraction systems, and vessels after use.
d. Re-inspect all equipment before use and line clearances at all stages of manufacture.
e. Ensuring all air conditioning systems are serviced and properly maintained.
f. Have a well-designed and operated facility.
g. Implement good housekeeping practices.
h. Develop written procedures for:
– Handling and storing material and products
– Cleaning of equipment and facilities
– Preventive maintenance programs
– Conducting room and line clearances
– How to train your personnel on GMP and specific duties
– Documenting and investigating all deviations or abnormalities

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.

Nice so much