Microbiology and Good Laboratory Practice in GMP
- Published on: Dec 16, 2020
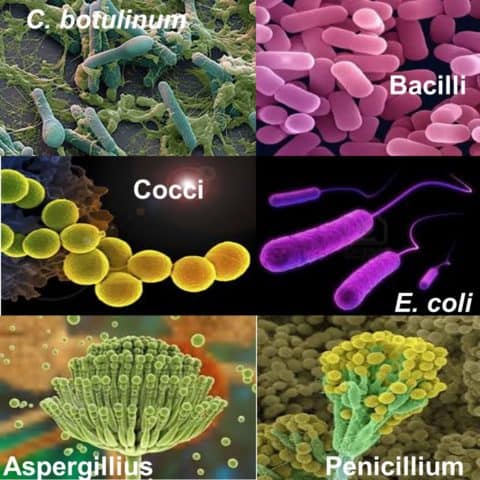
Defining micro-organisms
Micro-organisms are defined as living organisms that cannot be seen with the naked eye. The presence of micro-organisms not only has the potential to cause product degradation, but also to cause disease as the result of administration of the product.
Families:
 GLP Requirements
Role of the laboratory
The role of the microbiological quality control laboratory is to find, and sometimes enumerate and identify, micro-organisms present in samples.
Samples can be from:
GLP Requirements
Role of the laboratory
The role of the microbiological quality control laboratory is to find, and sometimes enumerate and identify, micro-organisms present in samples.
Samples can be from:
- Bacteria
- Fungi (yeasls and moulds)
- Algae
- Protozoa
- Cell has a nucleus
- Examples: yeasts, moulds, algae
- Cell does not have nucleus
- Examples: bacteria, archaebacteria
- Size
- Shape (e.g. rod, coccoid)
- Arrangement
- Cell wall structure (e.g. gram-positive, gram-negative)
- Oxygen requirements (e.g. aerobes, anaerobes, facultative anaerobes)
- Temperature requirements (e.g. mesophile, thermophile)
- Nutrient source (e.g. carbon, sugars)
- Biochemical reactions (e.g. ferment sugars to produce acid and gas)
- They can be potentially pathogenic to users, especially to immunocompromised patients
 GLP Requirements
Role of the laboratory
The role of the microbiological quality control laboratory is to find, and sometimes enumerate and identify, micro-organisms present in samples.
Samples can be from:
GLP Requirements
Role of the laboratory
The role of the microbiological quality control laboratory is to find, and sometimes enumerate and identify, micro-organisms present in samples.
Samples can be from:
- Sterile, pre-sterilisation, or non-sterile products
- Environment
- Raw materials, e.g. water
- Ancillary materials, e.g. lubricating fluids
- Preservative efficacy or effectiveness test (PET)
- Microbial limit testing
- Sterility
- Anti-microbial
- Bioburden program
- Growth promotion quality control (fertility)
- Bacteriostasis/fungistasis (stasis)
- Identification
- The sample needs to be representative of the batch. Problems can include micro-organisms multiplying and dying.
- The sample must accurately reflect the product, surface, or raw material.
- Check the sampling plan, technique, technique validation, and operator training and validation.
- Microbial contamination may not be evenly distributed within a container.
- The sample must be transported and stored so that the micro-organisms neither die nor multiply.
- Check the time the sample was taken, time the test commenced, the transport medias, and all temperatures.
- Log in and track the sample and storage conditions
- Correctly define and validate storage systems and requirements
- Assign the sample to a trained technician
- Training records should show test method competence, preferably validation
- Assign a test method correctly
- The method must be validated
- Monitor and document readings for validated incubators and refrigerators where samples are stored
- Assure that monitoring equipment is calibrated
- Equipment qualification/validation
- Thermal mapping of incubators
- Calibration and maintenance system
- Calibration of temperature monitoring devices for incubators
- Annual maintenance on heating and cooling system for incubators
- Test method validation and change control
- Documentation
- Training of staff
- Sample handling and storage
- Media batch records must be present
- Storage of media must be controlled
- Expiry date and date of manufacture must be recorded
- It must be QC tested
- pH, fertility, and sterility
- Media sterilisation must be validated and monitored for every batch
- Supporting documentation
- Traceability to the source (internal and external)
- Confirmatory testing supporting identification and purity
- Treat BIs as a raw material: apply QC to lots.
- Treat BIs as biological “contaminated”‘ material: uncontrolled dispersion may compromise manufacturing or QC testing.
- QC the BI count and identity.
- Control, issue, and reconcile usage, use, and storage.
- Require an SOP on BI handling and record system supporting use.
- Incubation time
- Temperature
- Medium used
- Disinfectants
- Bactericidal and bacteristatic agents
- Staff
- Staff garments
- Sampling equipment
- Sampling materials (e.g. media)
- Change outer garments
- Wash hands with disinfectant
- Change outer garments
- Wash hands with disinfectant
- Sanitise or sterilise any materials being transferred from the laboratory
- Well-defined flow that may be represented schematically
- Procedure control
- Strict labelling and storage requirements indicating material status, identification, and storage conditions
- Ensure storage is designated and segregated
- Contaminated materials and materials of unknown state or media do not cross the non-contaminated materials flow
- Contaminated materials and equipment must not contact items or documents that are non-contaminated or destined for outside the laboratory area
- registration and tracking
- storage
- processing
- incubation
- reading and recording
- results verification
- destruction
- Issues focused on by laboratory auditors
- Specific pharmaceutical regulatory requirements
- Sterility test features
- Identify different classes of micro-organisms
- Describe and list typical objectionable organisms for different products
- Follow correct procedures for safe disposal of cultures
- List the key attributes of the flow of samples and materials through the laboratory

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
You may like to read these related posts.
How to perform metal detection in pharmaceuticals processes
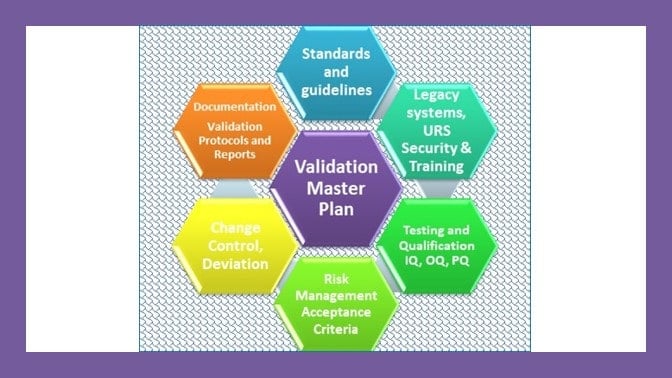
Nine steps for creating a Master Validation Plan
What is acceptable quality limit and how to use AQL in sampling